
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
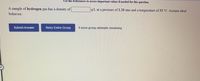
Transcribed Image Text:Use the References to access important values if needed for this question.
A sample of hydrogen gas has a density of
g/L at a pressure of 1.20 atm and a temperature of 53 °C. Assume ideal
behavior.
Submit Answer
Retry Entire Group
9 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A helium-filled weather balloon has a volume of 819 L at 14.9°C and 758 mmHg. It is released and rises to an altitude of 7.04 km, where the pressure is 372 mmHg and the temperature is –26.1°C. The volume of the balloon at this altitude is L. Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardce from Cengage Learning Submit Answer [Review Topics] What will the volume of a sample be if 301 mL of an ideal gas at 25 °C and 1.47 atm is cooled to 13 °C and 0.987 atm? Volume = mL Retry Entire Group [References] 8 more group attempts remaining E Assignmentsarrow_forwardSsigriment cise: Charles's Law O e liked this 0 discussions EXERCISE 1. Calculate the volume of a sample of nitrogen gas (N2) at 275°C if this sample has a volume of 10 L at 500°C and the pressure of the gas is constant. Enter your response here. Submit 9. I found this assignment helpfularrow_forward
- Could I hav help witrh this chemistry problemarrow_forwardUse the References to access important values if needed for this question. 1.07 mol sample of methane gas at a temperature of 21.0 °C is found to occupy a volume of 24.7 liters. The pressure of this gas sample is mm Hg. Submit Answer Retry Entire Group 8 more group attempts remainingarrow_forwardA cylinder of oxygen gas contains 54.4 g O2. If the volume of the cylinder is 6.67 L, what is the pressure of the O2 if the gas temperature is 22°C? Pressure atm An error has been detected in your answer. Check for typos, miscalculations etc. before submitting your answer. Submit Answer Retry Entire Group No more group attempts remainarrow_forward
- A mixture of krypton and helium gases, in a 6.73 L flask at 88 °C, contains 9.60 grams of krypton and 0.660 grams of helium. The partial pressure of helium in the flask is atm and the total pressure in the flask is atm. Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forward1- One mole of nitrogen and one mole of neon are combined in a closed container at STP. How big is the container?arrow_forwardWhat is the molecular mass of this gas? =______ g/mol?arrow_forward
- Question 18 of 25 A 20.0 L container at 303 K holds a mixture of two gases with a total pressure of 5.00 atm. If there are 2.22 mol of Gas A in the mixture, What quantity in moles of Gas B are present? tv A w 20 F3 000 O00 F4 F1 F2 F5 F6 F7 F8 @ # 2$ & * 1 2 4 7 8 Q W E R Yarrow_forwardA sample of oxygen gas has a density of| g/L at a pressure of 1.17 atm and a temperature of 38 °C. Assume ideal behavior. Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardView Policies Current Attempt in Progress How many moles of oxygen are in a 6250 mL sample of the gas at 932. mm Hg and 135 °C? mol eTextbook and Media Save for Later Attemarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY