
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:A sample of an ideal gas exerts 2.49 atm when it occupies 78.9 L. What will be the final pressure if
the volume decreases to 56.3 L at constant temperature?
3.49 atm
O 0.00460 atm
O 1.78 atm
O 0.287 atm
O 0.563 atm
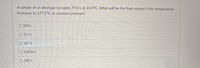
Transcribed Image Text:A sample of an ideal gas occupies 79.0 L at 24.0°C. What will be the final volume if the temperature
increases to 177.5°C at constant pressure?
O 584 L
O 52.1 L
O 10.7 L
O 0.0936 L
O 120. L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You fill a balloon with 1.15 L of air at 22°C and 0.850 atm pressure. You then take this balloon to a new location where the temper- ature is 34°C and you find that the volume has decreased to 0.963 L. What is the new pressure inside the balloon? 1. 0.980 atm 2. 0.905 atm 3. 0.975 atm 4. 1.06 atmarrow_forwardA sample of an ideal gas contains 0.697 moles. If the volume of the gas is 79.7 L and the temperature is 46.9 °C, what is the pressure of the gas? O 25.6 mmHg O 33.7 mmHg O 26.4 mmHg O 175. mmHg O 230. mmHgarrow_forwardA closed container holds1.31 moles of gas A0.660 moles of gas B1.64 moles of gas CIf the total pressure within the container is 4.08 atm, what is the partial pressure of gas C? 2.87 1.36 1.85 1.48 0.746arrow_forward
- The initial volume of an ideal gas is 3.0 L. If the pressure triples at constant temperature and constant moles, what is the final volume? 6.0 L 3.0 L 1.0 Larrow_forwardA 1.75 L container is filled with 235 g of argon gas. If the pressure is 75 atm, what is the temperature of the container? O 272 K O 6.8 K 9400 K 544 Karrow_forward43arrow_forward
- A sample of carbon dioxide gas at a pressure of 0.556 atm and temperature of 21.2 oC occupies a volume of 12.9 L. If the gas is allowed to expand at constant temperature to a volume of 22.3 L, what will be the pressure of the gas sample in atm? 0.322 atm 0.644 atm 0.961 atm 0.529 atmarrow_forward1. A cylinder contains 3.00 mol of Ar, 6.00 mol of Ne, and 1.00 mol of He. At 200 K, the total pressure is found to be 3.00 at What is the partial pressure of Ne gas in the cylinder? 01.80 atm 00.900 atm O2.00 atm 01.00 atm 18.0 atmarrow_forwardA sample of oxygen gas at 301 K and 2.02 atm occupies a volume of 1.82 L. If the gas is allowed to expand to a larger volume, while at the same time it is cooled to a lower temperature, the final gas pressure will be lower than 2.02 atm. O will be higher than 2.02 atm. could be higher or lower than 2.02 atm depending on the final volume and temperature.arrow_forward
- A sample of argon gas in an elastic container has a pressure of 2.0 atm and a volume of 4.0 L. If the pressure is increased to 3.75 atm, what will the new volume be?arrow_forward4arrow_forwardA balloon filled with a certain gas has a volume of 1.0 L and a pressure of 1 atm, what is the volume when the pressure is decreased to 0.80 atm? A sample of carbon dioxide gas has a volume of 5.0 mL at a temperature of 22.0 °C. What is the final volume of the sample when the temperature is 0.0°C?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY