
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please show your solution, thanks.
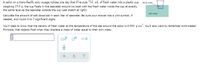
Transcribed Image Text:A sailor on a trans-Pacific solo voyage notices one day that if he puts 714. mL of fresh water into a plastic cup
fresh water
weighing 25.0 g, the cup floats in the seawater around his boat with the fresh water inside the cup at exactly
the same level as the seawater outside the cup (see sketch at right).
salt water
Calculate the amount of salt dissolved in each liter of seawater. Be sure your answer has a unit symbol, if
needed, and round it to 2 significant digits.
You'll need to know that the density of fresh water at the temperature of the sea around the sailor is 0.999 g/cm. You'll also want to remember Archimedes'
Principle, that objects float when they displace a mass of water equal to their own mass.
몸
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- Assume that a growth curve was done at a less-than-optimal salt concentration. Predict how the growth curve would change at a more optimal salt concentration. Explain your thinking.arrow_forwardAssuming that the standard solution contains 20.0 g/L of Red Dye #40, report the concentration in g/L of Red Dye #40 in your unknown. The path length is 1.00cm To test the unknown and standard solution, 0.1 mL of the unknown was dispensed into a 50-mL volumetric flask about three-fourths full with deionized water. this was done 1 more time for the unknown and two times for the standard solution. Therefore at the end, there were 4 flasks each with 0.1 mL of sample (2-unknown and 2-standard solution).arrow_forwardThanks in advance!arrow_forward
- Name: Date: Section: Prelaboratory Assignment: Determination of the Hardness of Water 1. A 0.512 g sample of CaCO, is dissolved in 12 M HCl and the mixture is diluted to 250 mL. a) How many moles of CaCO, are used? moles b) What is the [Ca²*] in the 250 mL solution? b) How many moles of Ca²* are in a 25 mL sample of the 250 mL solution? molesarrow_forwardCan you please answer 12.44 and do all of the sub problems and show the step by step to the solution pleasearrow_forwardA 1mL solution contains a 20X diluted sample. How do you make a spike solution that contains 100uL of the standard you will compare to the sample and 900uL of the diluting liquid? If you have a spike solution, do you still need to make a range of standard solutions? Please show detail, thank you.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY