
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
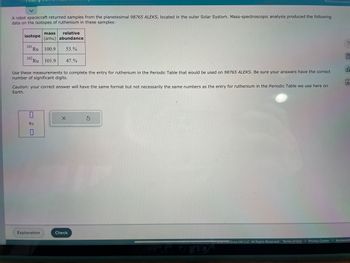
Transcribed Image Text:A robot spacecraft returned samples from the planetesimal 98765 ALEKS, located in the outer Solar System. Mass-spectroscopic analysis produced the following
data on the isotopes of ruthenium in these samples:
isotope
101 Ru
102 Ru
mass relative
(amu) abundance
100.9 53.%
Use these measurements to complete the entry for ruthenium in the Periodic Table that would be used on 98765 ALEKS. Be sure your answers have the correct
number of significant digits.
Ru
0
101.9 47.%
Caution: your correct answer will have the same format but not necessarily the same numbers as the entry for ruthenium in the Periodic Table we use here on
Earth.
Explanation
X
Check
S
zzz mcGraw Hill LLC. All Rights Reserved. Terms of Use
?
ol
Privacy Center | Accessibil
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- On planet X431A, the isotopes of carbon, CC , have the following natural abundances: IsotopeIsotope Abundance (%)Abundance (%) Mass (amu)Mass (amu) 12CX12X2212C 27.4527.45 12.00012.000 13CX13X2213C 22.8622.86 13.00313.003 14CX14X2214C 49.6949.69 14.00314.003 Determine the atomic weight of carbon on planet X431A. Atomic Mass:Atomic Mass: arrow_forwardAn element has three naturally occurring isotopes with the following masses and natural abundances: Isotope 1 2 3 mass (amu) % abundance 24.9858 23.9898 25.9825 10.13 79.13 10.74 Identify the element, give the name of the element.arrow_forward~ A robot spacecraft returned samples from the planetesimal 98765 ALEKS, located in the outer Solar System. Mass-spectroscopic analysis produced the follow data on the isotopes of tin in these samples: mass relative (amu) abundance 114 Sn 113.9 52.% 116 Sn 115.9 isotope esc Use these measurements to complete the entry for tin in the Periodic Table that would be used on 98765 ALEKS. Round your entry for the atomic mass to 3 significant digits. Caution: your correct answer will have the same format but not necessarily the same numbers as the entry for tin in the Periodic Table we use here on Earth. Explanation ! 0 Sn 0 1 Q A ? N @ 48.% 2 X Check W S #3 X H 1 option command 5 80 E D $ 4 C 999 000 R FL or dº V T G MacBook Pro 6 > Y B & 7 H 7 Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilit U N ▶II * α 8 J - M ▶ 9 K O ) ? A . 1 command optionarrow_forward
- Calculate the average atomic mass of silver using the following data:arrow_forwardCalculate the atomic mass of magnesium. The three magnesium isotopes, Mg-24, Mg-25, and Mg-26, have atomic masses and relative abundance of 23.985 amu (78.99%), 24.986 amu (10.00%), and 25.982 amu (11.01%) respectivelyarrow_forwardWhat volume of concentrated HCl (37.2% HCl m/m) contains 125 g HCl? The density of concentrated HCl is 1.19 g/mL.arrow_forward
- Please dont give hand written solutionarrow_forwardThe element uranium (U) has three naturally occuring isotopes, 234^U, 235^U, and 238^U. 234^U has an isotopic mass of 234.04 u and a relative abundance of 0.0054%. 235^U has an isotopic mass of 235.04 u and a relative abundance of 0.7204%. 238^U has an isotopic mass of 238.051 u and a relative abundance of 99.2742%. Calculate the average atomic mass of uranium.arrow_forwardThe newly-discovered "element ~X" has 3 stable isotopes, each with the listed mass and abundance. Determine what the atomic mass listed in the periodic table should be for "element~X", to at least 2 decimal places. Isotope Mass abundance 296X 296amu 85.7% 297X 297amu 10.4% 298X 298amu 3.9%arrow_forward
- suppose an element consisted of two isotopes with the masses 89.9 amu and 91.1 amu. If 1.500 x 10^-3 moles of this element is found to contain of 5.49 x 10^-4 moles of the isotope with the mass of 89.9 amu, what is the average atomic mass of this elementarrow_forward6. Calculate the atomic mass of a cosmic sample of silicon. The three silicon isotopes have atomic masses and relative abundances of 27.9769 amu (80.2297%), 28.9765 amu (12.6832%) and 29.9738 amu (7.0872%).arrow_forwardFictitious element, Fx, has two common isotopes. One of the isotopes has an atomic mass of 343.2 amu and a isotopic abundance of 33.60%. If the average atomic mass of Fx is 345.9 amu, calculate the atomic mass (in amu) of the other isotope.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY