A rigid storage tank contains 30 (kg) of ideal hydrogen (M=2.016 kg/kmol). During a fire, heat (128.5 MJ> Mega Joules) is transferred to the tank. The initial temperature and pressure are 300 (K) and 500 (kPa), respectively. The burst pressure of tank is 1.5 MPa. Will the tank rupture? Ignore kinetic and potential effects. kJ/kmol.K. The value of universal gas constant is 8.314
A rigid storage tank contains 30 (kg) of ideal hydrogen (M=2.016 kg/kmol). During a fire, heat (128.5 MJ> Mega Joules) is transferred to the tank. The initial temperature and pressure are 300 (K) and 500 (kPa), respectively. The burst pressure of tank is 1.5 MPa. Will the tank rupture? Ignore kinetic and potential effects. kJ/kmol.K. The value of universal gas constant is 8.314
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter2: Matter And Energy
Section: Chapter Questions
Problem 29RQ: If 3000 ft3 of air is crossing an evaporator coil and iscooled from 75F to 55F, what would be the...
Related questions
Question
Qsafe = 187.586 MJ

Transcribed Image Text:2. A rigid storage tank contains 30 (kg) of ideal hydrogen (M=2.016 kg/kmol). During a fire, heat (128.5 MJ→ Mega Joules) is
transferred to the tank. The initial temperature and pressure are 300 (K) and 500 (kPa), respectively. The burst pressure of tank
is 1.5 MPa. Will the tank rupture? Ignore kinetic and potential effects.
kJ/kmol.K.
The value of universal gas constant is 8.314
Expert Solution
Step 1
The given data are:
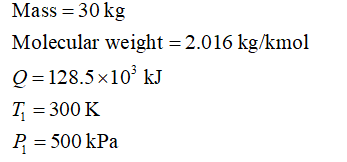
Step 2
As it mentions the tank is rigid, therefore the volume is constant.
Now apply the first law of thermodynamics.
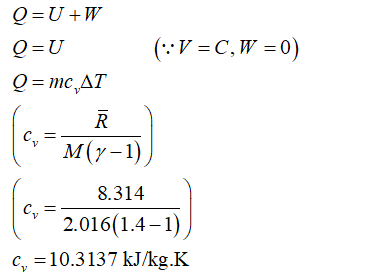
Step by step
Solved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning