
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:A Review Constants Periodic
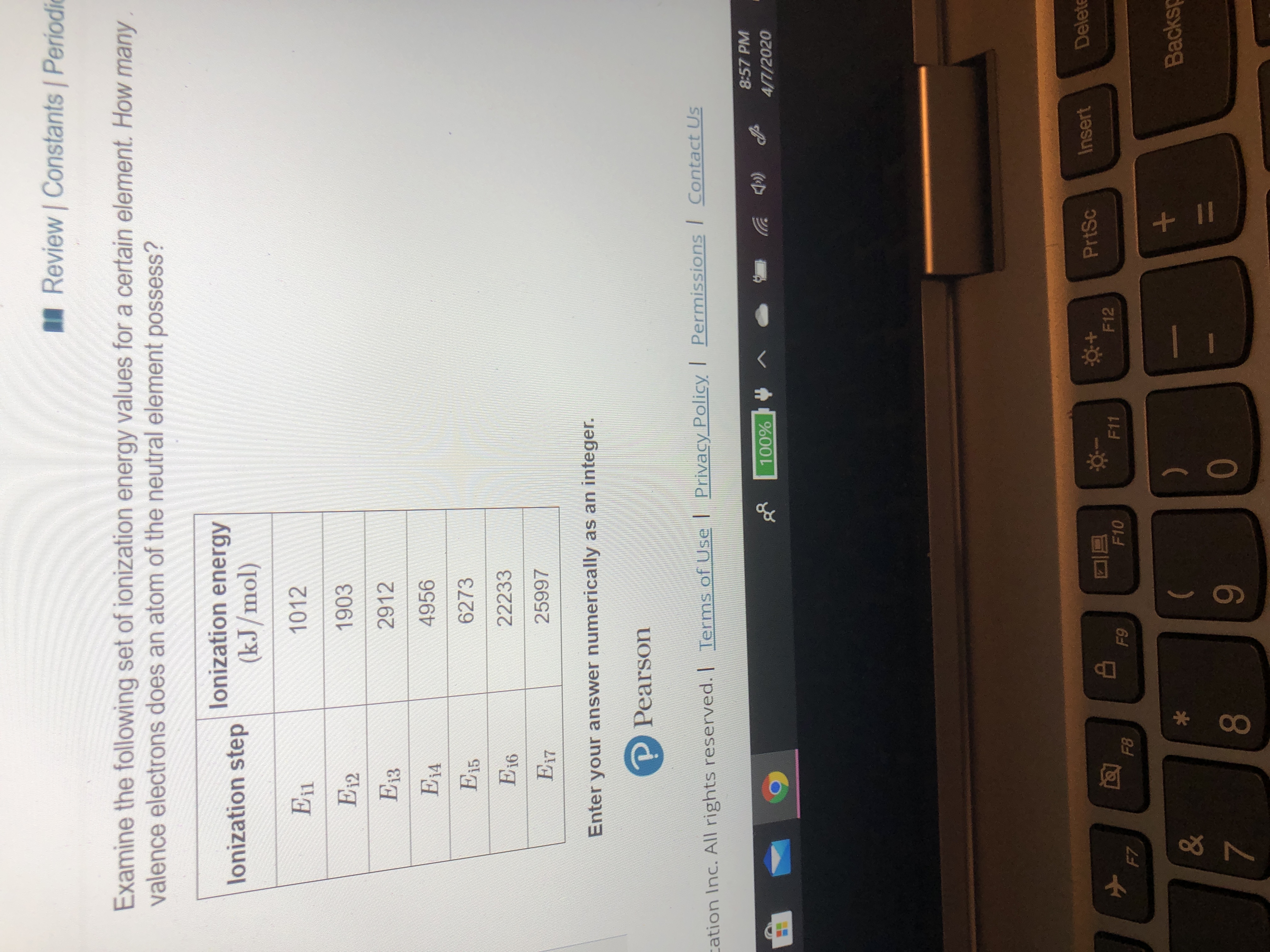
Examine the following set of ionization energy values for a certain element. How many
valence electrons does an atom of the neutral element possess?
lonization energy
lonization step
(kJ/mol)
En
1012
E12
1903
Ез
2912
E14
4956
|E15
6273
E16
22233
|E17
25997
Enter your answer numerically as an integer.
Pearson
cation Inc. All rights reserved. I Terms of Use | Privacy Policy Permissions Contact Us
8:57 PM
100%
4/7/2020
PrtSc
Insert
Delete
F12
F10
F11
F8
F9
F7
Backsp
&
7
80
9
+II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Similar questions
- Please helparrow_forwardELEMENTS: Ca, K, Al, Mg, S, O, P, F Now put 8 different possible combinations together. Write the formulas below. Make sure total charge = 0 Examples that work: (do NOT copy these) CaS CaF2 Non-example: CaKParrow_forwardRefer to the following figure to answer the questions below. Atomic mass Atomic number 12 16 6 1. 14 32 31, 16 15 How many electrons will a single atom of sulfur with no charge and no bonds have in its valence shell? 32 8 16 6arrow_forward
- Which ion is incorrect? 02 O K* Sr2+ 02 O Mg2+ S3-arrow_forward17.arrow_forwardWhen sodium reacts with chlorine to form an ionic compound, each metal atom loses _____ electron(s) and each nonmetal atom gains _____ electron(s). There must be ___(1,2,3) sodium atom(s) for every ___ (1,2,3) chlorine atom(s) in the reaction.arrow_forward
- Sharing Covalent bonding is explained as a bond that forms when atoms are electrons. When the two atoms get close enough, the nucleus of one atom weakly attracts the other atom's (Protons / Neutrons / Electrons ). When the two atoms get even closer, the nucleus of one atom has a strong attraction for both its_uclei and the electron from At this point both atoms are attracting ● You can see that the electrons spend almost all their time the two nuclei. When showing the attractions from another nucleus you can see that it is also pulling on both electrons. This is a bond. The atoms are both trying to one can take them away from the other. This creates a situation where both atoms are together. What region of the periodic table are their atoms that attract electrons strongly? Non Metals the same electrons, but neither H Barrow_forwardA monatomic ion with a charge of -2 has an electronic configuration of 1s22s22p63s23p64s23d104p65s24d105p6.This ion is a(n) _______cation anion.What is the chemical symbol of the noble gas this ion is isoelectronic with? Xe.What is the formula of the ion? Ba2+.arrow_forwardDetermine the number of valence electrons for each of the atoms. Enter each answer as a numeral. For example, if an atom has two valence electrons, enter the number 2. B: Mg: O: Xe:arrow_forward
- O east.cengagenow.com e: CHM 103_003 General Chemistry I C OWLV2 | Online teaching and learning resource from Cengage Learning 5:08 [References] Which of the following groups contains no ionic compounds? O CH,O, H2S, NH3 O KOH, CCI4, SF4 O HCN, NO2, Ca(NO3)2 O NaH, CaF2, NANH2 O PCI5, LiBr, Zn(OH)2 1 pt 1 pt 1 pt 1 pt 1 pt 1 pt Submit Answer Try Another Version 5 item attempts remaining 1 pt 1 pt 1 pt 1 pt nent Cengage Learning Cengage Technical Supportarrow_forwarda. If an atom of sodium contains 1 valence electron and an ion of sodium contains 8 and has a 1+ charge, what did the sodium atom do to become a sodium ion? b. If an atom of oxygen contains 6 valence electrons and an ion of oxygen contains 8 and has a 2− charge, what did the oxygen atom do to become an oxygen ion?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY