
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
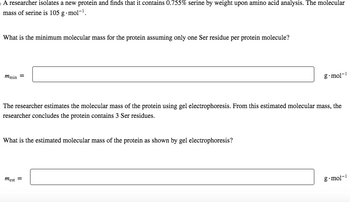
Transcribed Image Text:A researcher isolates a new protein and finds that it contains 0.755% serine by weight upon amino acid analysis. The molecular
mass of serine is 105 g.mol-¹.
What is the minimum molecular mass for the protein assuming only one Ser residue per protein molecule?
mmin =
The researcher estimates the molecular mass of the protein using gel electrophoresis. From this estimated molecular mass, the
researcher concludes the protein contains 3 Ser residues.
What is the estimated molecular mass of the protein as shown by gel electrophoresis?
g.mol-¹
mest =
g.mol-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Similar questions
- What is the minimum length of the column required to completely separate the amino acids? What is the time required for solvent elution (i.e. time for all amino acids to exit the column)?arrow_forwardJacqueline isolated an interesting new protein from the broth of a yeast culture. After purification of the protein, she requested an amino acid analysis and found that the protein contained 0.57% tryptophan by weight (tryptophan Mr-204). Calculate the minimum molecular weight of the protein (i.e., assuming there is only one tryptophan residue per protein molecule).arrow_forwardA gel filtration column with a fractionation range of 1.5-20 kDa is used to separate out the proteins shown below. If these proteins are collected into separate fractions in between the void volume and total volume, in which order will they elute? Indicate if any of the proteins are found in the void volume or total volume fractions. Protein Z - 3330Da Protein Y - 13kDa Protein X - 1.3kDa I. Total volume fraction II. Third protein fraction III. Second protein fraction IV. First protein fraction V. Void volume fractionarrow_forward
- Marker Proteins Molecular Weight (daltons) Distance 132,000 66,000 43,000 14,400 Serum Albumin (Dimer) Serum Albumin (Monomer) 23mm 32mm Ovalbumin 39mm 60mm Lysozyme Unknown 1 26mm Unknown 2 58mm Rabbit serum (albumin) 33mm 1.On semilog paper (provided below) plot the distance migrated by each standard protein as a function of molecular weight. Determine the molecular weight of the unknown proteins and the major protein found in rabbit serum (albumin) from this calibration curve. ii) Interferon is a protein of 25,000 Dalton. How far would it have migrated, land it been included in this experiment? iii) The average amino acid residue in a protein has a molecular weight of 120 Daltons. Assuming that your unknowns are average, how many amino acid residues do they containarrow_forwardYou have a soluble protein that is highly flexible and is only 23 kDa in size. What is the most suitable technique (X-ray crystallography, NMR, cryo-EM) for structure determination of this protein? Explain your reasoning.arrow_forwardYou perform electrophoresis of 3 proteins, Hemoglobin (pl: 6.8), DNA Polymerase (pl: 5.2) and Porcine pepsin (pl: 1.0) in a Tris-Glycine buffer (pH 5.2). (pl = Isoelectric point). Select which of the following is true. O All proteins will migrate at the same rate O All proteins will migrate at the same direction Porcine pepsin will migrate toward the electrode Porcine pepsin will not migrate and stay in the wellarrow_forward
- In RP HPLC, one of the most common separation methods used to measure purity, strength, dosage, etc, a protein would be put into 0.1% (1000 ppm) TFA (Trifluoroacetic acid), what do you suppose this does to the protein in many cases? (Pick the BEST answer). somewhat denaturing to very denaturing oxidizes cysteine ionizes acid and base groupsarrow_forwardFour proteins Cytochrome C (pI=10.2) Myoglobin (pI=7.2), Hemoglobin (pI = 6.8) and Serum Albumin (pI= 4.8) were used in our gel electrophoresis lab exercise. What is the molecular basis for the differences in the electrophoretic mobilities of the four proteins analyzed in this exercises? size shape charge all of themarrow_forwardYou assay 0.5 ml of 1/2000 diluted egg white for protein, as described in the practical schedule, and the spectrophotometer reading at 595 nm is 0.6. Using the attached calibration curve(click to enlarge), calculate the concentration of protein in undiluted egg white. Give your answer in mg/ml.arrow_forward
- Calculate the Rf values for each of the amino acids and enter your data in the table.Based on your calculations, what is the identity of the Unknown amino acid? Rank the amino acids from most hydrophilic to most hydrophobicarrow_forward1.0.1 mL of a protein solution of concentration of 11 mg/mL was diluted to a total volume of 4.0 mL with water (i.e. 0.1 mL of the solution was added to 3.9 mL of water). 2 mL of this solution was then mixed with 18 mL of water. What is the concentration of the diluted protein solution? Space to show your workings:arrow_forwardCO2 Iragment (interinediary) to slaction transport chain CH Pyruvate -Krebs/citric acid cycle COA COO CH C - S CH2 HO C CO" CH2 Acetyi CoA CH COO CH2 HS-COA H0-CH NADH HC CH2 NAD+ Oxaloacetate Citrate HO-CH Isocitrate NAD+ Malate NADH CO H20- CH a-Ketoglutarate CH2 HC NAD+ Fumarate NADH CH2 COO O2 FADH FAD Suceinyl CoA Succinate COO GTP GDP CO" 1. CH: PO. CH2 CH2 CH2 GTP 1. S COA GDP COO ADP ATP iii. An acyl-group transfer can be seen twice in the cycle. The first time when -SCOA group on acetyl- COA is replaced with OH from water (which then isarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON