
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
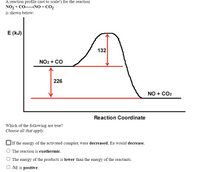
Transcribed Image Text:A reaction profile (not to scale!) for the reaction
NO, + CONO + CO,
is shown below:
E (kJ)
132
NO2 + CO
226
NO + CO2
Reaction Coordinate
Which of the following are true?
Choose all that apply.
JIf the energy of the activated complex were decreased, Ea would decrease.
The reaction is exothermic.
O The energy of the products is lower than the energy of the reactants.
O AE is positive.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ni (s) + 4 CO (g) Ni(CO)4 (g) In which direction will the reaction shift if the reaction was heated on a hotplate?arrow_forwardQuestion Completion Status: QUESTION 7 The following reaction is exothermic: CF2C12(g) CF2CI(g) + Cl(g) Select the energy diagram that correctly shows the relative energies of the reactants, products, and the activated complex as well as the correct molecular representations of reactants, products, and possible structure for the activated complex. Eproducts E. EReactants Reaction Progress EProducts E, EReactants Reaction Progress Eproducts Ea EReactants Reaction Progress EProducts Ea EReactants Save All Arnswers Reaction Progress Click Save and Submit to save and submit. Click Save All Answers to save all answers. Energy Energy Energyarrow_forwardk=? k=?arrow_forward
- What is the concentration of A after 37.5 minutes for the reaction A → Products when the initial concentration of A is 0.750 M? (k = 0.0451 M⁻¹min⁻¹)arrow_forwardConsider the quilibrium reaction between X and Y, as shown below: X=Y AG The reaction is started with 10 mmol of X; no Y is initially present. After 48 hours, analysis reveals the presence of 10 mmol of X and 0 mmol of Y. Which is the most likely explanation? = −1 - 45 kJ mol X and Y have reached equilibrium concentrations. An enzyme has shifted the equilibrium toward X. Formation of Y is kinetically slow; equilibrium has not been reached by 48 hours. Formation of Y is thermodynamically unfavorable. Two of the above explanations are reasonable.arrow_forwardWhat is the concentration of A after 30.5 minutes for the reaction A → Products when the initial concentration of A is 0.750 M? (k = 0.0451 M⁻¹min⁻¹)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY