
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
In each of the following three reaction coordinate diagrams, state:
Q) Whether the reaction is exothermic or endothermic
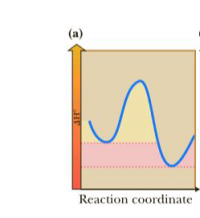
Transcribed Image Text:(a)
Reaction coordinate
HV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4:47 Question 2 of 13 When a system is at dynamic equilibrium, A) no reactions are occurring. B) a reaction is occurring in only one direction. Submit C) the rates of the forward and reverse reactions are equal. D) all of the reactants have been converted to products Tap here or pull up for additional resourcesarrow_forwardHow does a combination reaction differs from a descomposition reaction?arrow_forwardImagesarrow_forward
- What may happen to the reaction rate when you add catalyst on it? Choose from the choices below: It increased It decreased, then increased It decreased It did not changearrow_forwardPlease provide only typed answer solution no handwritten solution needed allowedarrow_forwardWHAT IS A POSDIBLE REACTION OF AN ENDOTHERMIC TO INCREASED ENVRONMENTAL TEMPERATURESarrow_forward
- 1. Define each of the following for a chemical reaction: Favored Non-favored Exothermic Endothermic AH AEarrow_forwardOne proposed source of clean energy is the combustion of hydrogen in the presence of oxygen. This reaction releases a significant amount of energy, and the only product of the reaction is water. The balanced chemical equation is shown here: 2H₂(g) + O₂(g) →→→ 2H₂O(g) + energyarrow_forwardConsider the relative energy diagrams for four different processes: Potential energy A Reaction coordinate Potential energy B Reaction coordinate Potential energy Reaction coordinate Compare energy diagrams A and B. Which process will more greatly favor products at equilibrium? Explain. Process B more greatly favors products at equilibrium because it has a lower energy of activation. Potential energy Process A more greatly favors products at equilibrium because it has a higher energy of activation. Process A more greatly favors products at equilibrium because it is exergonic. Process B more greatly favors products at equilibrium because it is endergonic. D Reaction coordinatearrow_forward
- The dissolving of ammonium nitrate is often used in an instant cold pack used to relieve swelling. If this process is an endothermic reaction, i.e. absorbs heat, then why does it feel cold to the touch instead of hot?arrow_forwardA reaction where the products are higher in energy than the reactants is an example of an exothermic process endothermic process exergonic processarrow_forwardConsider the following reaction for this question Cl₂, H₂Oarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY