
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
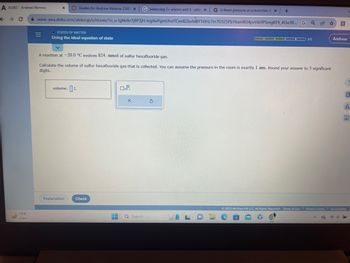
Ideal equation of state, easy one
Transcribed Image Text:A ALEKS-Andrew Herrera
← → C www-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvgXwPgmUhvITCeeBZbufuBYTi0Hz7m7D3ZSPbY6amBU4pvVib9PSmgKF9_4Uw3R... Ga
73°F
Clear
=
X
O STATES OF MATTER
Using the ideal equation of state
Grades for Andrew Herrera: CHEN X G balancing 2+ anions and 3- catio x QIs there pressure at universities fc x +
X
volume:
Explanation
A reaction at -20.0 °C evolves 824. mmol of sulfur hexafluoride gas.
Calculate the volume of sulfur hexafluoride gas that is collected. You can assume the pressure in the room is exactly 1 atm. Round your answer to 3 significant
digits.
Check
▬▬
0.8.
X
Q Search
▬▬▬▬▬
Ś
3/5
h
Co
Andrew
EBEN
olo
Ar
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated water bath. (See sketch at right.) 1 atm pressure piston cylinder From previous experiments, this chemical reaction is known to absorb 307. kJ of water bath gases energy. The position of the piston is monitored, and it is determined from this data that the system does 103. kJ of work on the piston during the reaction. Ar exothermic Is the reaction exothermic or endothermic? endothermic up Does the temperature of the water bath go up or down? down neither in Does the piston move in or out? out neither in Does heat flow into or out of the gas mixture? out neither How much heat flows? Be sure your answer has the correct number of significant digits.arrow_forwardS value is in the tablearrow_forwardUsing the ideal equation of state A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 52.0 cm wide and 62.4 cm high. The maximum safe pressure inside the vessel has been measured to be 6.60 MPa. For a certain reaction the vessel may contain up to 22.4 kg of chlorine pentafluoride gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Round your answer to 3 significant digits. temperature: c Explanation Check x10 80 X Ś F6 azinsight.adsrvr.org/track/clk?imp=fbec12b0-7f3d-4127-929d-a59782e61fbb&ag=r735012&sfe=1788cd47&sig=PCK7iCABpePPZo19wV_E9gKn0Pi6FOTr7R3rMUmfWw.&crid=2zvfx50b&cf=5773664&fq=0&t=2&td_szwww.chegg.com 8. 15 F7 W Danasia V DII dloarrow_forward
- Please correct answer and don't use hand ratingarrow_forwardPlease correct answer and don't use hend raitingarrow_forwardFrom the following vapor pressure data for diethyl ether, an estimate of the molar heat of vaporization of C2H5OC2H5 is P, mm Hg T, K kJ/mol. 100. 262 400. 291 Submit Answer Retry Entire Group 7 more group attempts remainingarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY