
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
a.) Determine the mass flow rate of the water vapor, in kg/hr.
b.) Calculate the volumetric flow rate of the water vapor leaving the plant, in m^3/hr.
c.) Calculate the volumetric flow rate of the brine, in m^3/hr.
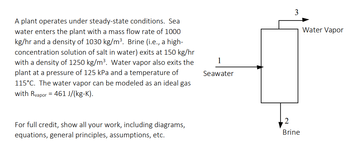
Transcribed Image Text:A plant operates under steady-state conditions. Sea
water enters the plant with a mass flow rate of 1000
kg/hr and a density of 1030 kg/m³. Brine (i.e., a high-
concentration solution of salt in water) exits at 150 kg/hr
with a density of 1250 kg/m³. Water vapor also exits the
plant at a pressure of 125 kPa and a temperature of
115°C. The water vapor can be modeled as an ideal gas
with Rvapor = 461 J/(kg-K).
For full credit, show all your work, including diagrams,
equations, general principles, assumptions, etc.
Seawater
3
,2
Brine
Water Vapor
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 1. If 1 gallon of oil = 1.4 therm, how many gallons have been wasted by heat loss in Feb? (1 therm = 100,000 BTU)_____________________________________________________________________________________2. If $1.30 per therm, how much did you waste from house heat loss in Feb?arrow_forward.- Air is cooled and humidified, initially at 90 °F BS and 70 °F BH is cooled and dehumidified, reaching 56 °F BS and 54 °F BH, 3000 CFM is supplied. Calculate: a) sensible heat b) latent heat c) total heat d) Lbs. moisture removed per hour e) determine the RH at the inlet and outletarrow_forwarda) Find the cross-sectional area of the incoming jet (in cm^2) b) Find the horizontal force on the block (in Newtons) (Note: remember to convert the area of the jets from cm^2 to m^2!) c) The vertical force on the block is found to be Fy. What velocity of the incoming jet (i.e. what value of u1) would be needed to generate a force of 4Fy? (Note that you don't need to actually find Fy to solve this question!)arrow_forward
- b) A 3 cm orifice plate is placed within a 4 cm pipe in which methanol at 20 °C (SG = 0.7884 and dynamic viscosity (µ) = 0.5857 cP) is flowing through. If the flow rate passing through the pipe is 3.1 litres per seconds, determine the pressure difference that must be measured around the orifice plate. The discharge coefficient of the orifice can be calculated by: 91.71B2.5 Ca = 0.5959 + 0.0312B2.1 – 0.184ß® + Re0.75 Where, B is the ratio of orifice diameter to pipe diameter and Re is the Reynolds number.arrow_forwardHydraulic motor calculations Theoretical flow = 1.5 GPMRPM output = 100 RPMMechanical power = 0.25 HP Total efficiency = 85%Volumetric efficiency = 92% a) Calculate the theoretical engine displacement (in3/r). b) Calculate the actual engine flow in GPM. c) Calculate the actual torque (lb.in).arrow_forwarda) A suction pump. Consider a pipe that is inserted vertically into water. By suction, the pipe is evacuated of air. How high will the water rise? b) A water turbine is designed to extract kinetic energy from the hor- izontal flow of water and convert it into rotation of the turbine. You may assume that the height of the fluid does not change as it flows through the turbine, and that the fluid is incompressible. If the velocity is lowered from 100 m/s to 70 m/s as the fluid flows through the turbine, what is the change in pressure? c) Is the pressure higher upstream or downstream? d) The fluid in (b) is replaced by air to make a wind turbine. What is the change in pressure now for the identical change in velocity to (b)?arrow_forward
- Please explain all intermediate steps. Thanks youarrow_forwardA new type of energy absorber is being designed as a buffer at the end of track at a fairground.It consists of a piston with small holes that moves in a cylinder containing oil, so that the kineticenergy of impact is absorbed as heat by the oil.a) Draw a sketch for the instant of impact by a vehicle of mass 2500kg moving at 30mphshowing the forces and energy transfers involved.b) Write down the first law of thermodynamics for a system and identify terms that are notrelevant if the oil is taken as the system.c) How much heat transfer to the surroundings is required to return the oil to its originaltemperature after an impact by a 2500kg vehicle moving at 30mph?arrow_forwardA pipe has a cross-sectional area of 16.5cm2 at point A, where the speed of the fluid (water) is 1.2 m/s and the pressure is 2.00 atm. The pipe ascends 3.00 m and narrows to 8.20 cm2 at point C. a) what's the speed of the fluid at point C. b) what's the absolute pressure at point C.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY