Question
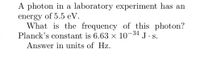
Transcribed Image Text:A photon in a laboratory experiment has an
energy of 5.5 eV.
What is the frequency of this photon?
-34
Planck's constant is 6.63 x 10°
J.S.
Answer in units of Hz.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Find the energy of the following. Express your answers in units of electron volts, noting that 1 eV = 1.60 10-19 J.(a) a photon having a frequency of 7.20 1017 Hz eV (b) a photon having a wavelength of 5.00 102 nm eVarrow_forwardAccording to the Bohr model of a hydrogen atom, the frequency of light radiated by an electron moving from an orbit ₁ to an orbit n₂ corresponds to the energy level difference between ₁ and ₂ of where E = Eo ( − 2) - mez²4 32x²² Eo and where me is the electron mass, Z is the atomic number, e is the magnitude of the electron charge, is the permittivity of free space, and ħ is Planck's constnt divided by 27. In the case of hydrogen (Z = 1) Eo = -13.6 eV. == Part A Find the frequency of light f radiated by an electron moving from orbit ₁2 to n₂ = 1 inside of a He+ ion. Express your answer in hertz to three significant figures. ► View Available Hint(s) f= 15| ΑΣΦ Submit Part B ? Hzarrow_forwardIf an excited state of an atom has a lifetime of 3.0××10^−7 s, what is the minimum error associated with the measurement of the energy of this state? ΔE=____ ×10^−28 Jarrow_forward
- An electron at the n=5 energy level of hydrogen undergoes a transition to the n=3 energy level. What wavelength of photon does the atom emit in this process? a) 1.28 x 10^-6 m b) 2.37 x 10^-6 m c) 4.22 x 10^-7 m d) 3.04 x 10^-6 m e) 5.92 x 10^-5 marrow_forwardA ball has a mass of 0.216 kg just before it strikes the Earth after being dropped from a building 74.1 m tall. What is its de Broglie wavelength? The acceleration of gravity is 9.8 m/s 2 and Planck’s constant is 6.62607 × 10−34 J · s. Answer in units of m.arrow_forward1. A cube of iron (atomic number 26), whose side measures 1 ft (30.48cm), is floating in a dark space. It is observed to emit blackbody radiation at the power of 200oW. (a) Would you dare to touch it for $20? Find the temperature of the cube before you answer. (b) Find the peak frequency of the spectrum, and compute the energy of photon at the frequency. Give the energy in units of electron-volt (eV). (c) What would be the temperature for the radiation if the cube were made of alu- minum (atomic number 13)?arrow_forward
- When you talk about FM radio stations and talk about the frequency it emits at, you are talking about the frequency of the electromagnetic wave that leaves the station. Traditionally radio stations are measured in MHz, where 1MHz = 106 Hz. If a FM radio station is at 93.8 MHz, what is the energy in Joules of the photon associated with this radio wave? Planck's Constant is 6.63 x 10-34 J*sarrow_forwardA photon has an energy of E1= 1.0x10^-20 J. A second photon has twice the wavelength as the first photon. Which of the following regarding the second photon is true? A. E2= (1/2)E1 B. E2= E1 C. E2= 2E1 D. E2= 3E1arrow_forwardLight of frequency 2.1 × 1015 Hz illuminates a piece of cesium, and the cesium emits photoelectrons with a maximum kinetic energy of 6.5 eV. What is the threshold frequency of the metal? Planck’s constant is 6.63 × 10−34 J · s. Answer in units of Hz.arrow_forward
- The mass of an electron is 9.11 10-31 kg.A.) If the wavelength of an electron is 5.02 10-7 m, how fast is it moving? B.) If an electron has a speed equal to 4.30 106 m/s, what is its wavelength?arrow_forward3) What is the wavelength of a photon in nanometers, having energy of 2.00 eV? (e-3.00 - 10 m/s, h = 6.626 x 10-34 J-s, 1 eV = 1.60 × 10-19 J) s)arrow_forward3. A. 279.7 kJ of energy are required to remove one mole of electrons from one mole of lithium atoms. What is the maximum wavelength of light that can remove one electron from one lithium atom? (h = 6.626 x 1034J•sec, c = 3.0 x 10* m/s) B. Assume that a hydrogen atom's electron has been excited to the n= 5 energy level. How many different wavelengths of light can be emitted as this excited electron loses energy? Include a diagram to support your answer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios