
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
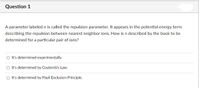
Transcribed Image Text:Question 1
A parameter labeled n is called the repulsion parameter. It appears in the potential energy term
describing the repulsion between nearest neighbor ions. How is n described by the book to be
determined for a particular pair of ions?
O It's determined experimentally.
O It's determined by Coulomb's Law.
O It's determined by Pauli Exclusion Principle.
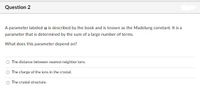
Transcribed Image Text:Question 2
A parameter labeled a is described by the book and is known as the Madelung constant. It is a
parameter that is determined by the sum of a large number of terms.
What does this parameter depend on?
The distance between nearest neighbor ions.
The charge of the ions in the crystal.
O The crystal structure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the electron configuration for an Al atom. Also, identify which of these electrons are valence electrons.arrow_forwardConsider the following orbital energy diagram, which shown where electrons reside within atomic orbitals. Select ALL of the following atoms or ions that could be represented by this orbital energy diagram. Group of answer choices F- (fluoride ion) Ne atom C atom O2- (oxide ion) Br- (bromide ion) Li atom 1s full, 2s full, and 3 rows of 2p fullarrow_forwardHow many valence electrons would be in carbon, copper, cobalt, argon and potassium?arrow_forward
- Give the noble gas electron configurations and draw the valence orbital diagramsfor each of the following atoms or ions.a. Cub. V^2+c. Te^3-d. Warrow_forward5. Isoelectronic ions are the species that have the same number of electrons. Part a. What +2 ion is isoelectronic with Na+? What -2 ion? Part b. Which of the following has (have) the same electron configuration as (isoelectronic with) Ar? A. K- B. Ca2+ C. 02- D. CI-arrow_forwardWrite a chemical equation representing the third ionization energy for lithium. Use e− as the symbol for an electron.arrow_forward
- It takes 242. kJ/mol to break a chlorine-chlorine single bond. Calculate the maximum wavelength of light for which a chlorine-chlorine single bond could be broken by absorbing a single photon. Be sure your answer has the correct number of significant digits. nm 0 10 Xarrow_forward8.Pick four elements in the main group (representative element) of the fourth period. a.Write its abbreviated electron configuration. b.Write the correct formula for its oxide. If it forms more than one oxide, you may list them all, but it is not necessary. If it does not form an oxide, state that. c.Is the most common ion the element forms larger or smaller than its neutral atom? Explain your answer in terms of electronic shells/subshells.arrow_forwardExplain the advantages and disadvantages of electron impact ion Soures (EIS)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY