Question
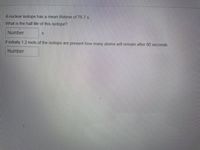
Transcribed Image Text:A nuclear isotope has a mean lifetime of 76.7 s.
What is the half life of this isotope?
Number
If initially 1.2 mols of the isotope are present how many atoms will remain after 80 seconds
Number
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- QUESTION 9 _was the first to calculate how much more energy an atom release in radioactive decay that it does in any chemical reaction. J.J.Thompson W.Roentgen Marie Curie O F. Soddyarrow_forwardA 10 g sample of a radioactive element took 12 days to reduce into 1 g. Calculate the half-life and identify the element.arrow_forwardAnswer the following questions regarding the radioactive isotope radium-226. A.Write a nuclear equation for when this nucleus undergoes beta decay. B.What is the binding energy of a radium-226 nucleus? The mass of radium-226 is 226.02541 amu. C.A rock obtained by geologists was found to obtain 1.38 mg of radium-226. Later studies confirmed that 922 years prior, the rock contained 34.6 mg of radium-226. How many half-lives have elapsed? D.Based on your answer to Question C, determine the half-life of radium-226. E.Based on your answers to Questions C & D, determine the rate constant of the beta decay of radium-226arrow_forward
- Radioactive element AA can decay to either element BB or element CC.The decay depends on chance, but the ratio of the resulting number of BB atoms to the resulting number of CC atoms is always 2/1.The decay has a half-life of 8.00 days.We start with a sample of pure AA. How long must we wait until the number of CC atoms is 1.50 times the number of AAatoms?arrow_forwardThe half-life of radium-226 is 1.6 x 10 years. How long will it take for 20.0 mg of radium-226 to decay to 2.50 mg? 1.3 x 103 1.6 x 10' years A. years B. 3.2 x 10 years 103 С. D. 4.8 x yearsarrow_forwardA 5 g sample of a radioactive element takes 102 days to reduce into 3 g. Calculate the half-life and identify the element.arrow_forward
- What is the mass of 25.0 mCi of technetium-99m (Tc99m)Tc99m , which has a half-life of 6.00 h?arrow_forwardA sample of carbon-14 initially consists of 5 × 10 24 particles. Carbon-14 has a half- life of 5730 years. a. What is the decay constant for carbon-14? (Answer in units of yr –1 .) b. How many radioactive particles of the sample remain after 100 years? c. What percentage of the radioactive particles remains after 500 years? d. How many radioactive particles of the sample remain after 1000 years? e. How much time will it take for 50% of the particles to decay? f. How much time will it take for 99% of the particles to decay? g. How many half-lives will it take for 99% of the sample to decay? h. What is the initial decay rate of the sample? (Answer in decays/yr.) i. After 200 years, what is the decay rate of the sample? j. How long will it take for the decay rate to decrease to 1015 decays/year? k. How many half-lives have passed after the time you found in part (j)?arrow_forwardQuestion A8 Sketch a diagram indicating the s-process path from 131 Xe to 140 Ce. Specify the type of each process (i.e. neutron capture, electron capture, beta decays) in the path.arrow_forward
- RADIOACTIVITY. LAW OF RADIOACTIVE DECAY Activity units: 1 Ci = 3.7 × 1010 Bq 1. Nuclear decay law: N = Noe-At The law of radioactive decay gives the quantitative relationship between the original number of nuclei present at time zero No and the number N at a later time t (sec), where e = 2.71828... is the base of the natural logarithm, and 2 is the decay constant for the nuclide (1/s). 2. Relationship between decay constant 1 and isotop half-life T1/2: In(2) 0.693 Modality Radioisotope Carbon-11 Half-life T1/2 where 1 is decay constant (1/s), T1/2 is isotope half- life (s). T1/2 20 minutes PET Fluorine-18 110 minutes PET 12.7 hours Copper-64 Gallium-67 PET 3. Activity or decay rate of a radioactive source A: ΔΝ 78.3 hours SPECT Gallium-68 68 minutes PET = AN Technetum-99m 6.02 hours SPECT Δt where A is activity or decay rate of a radioactive source (Bq), N is number of nuclei present at time t (s ), A is decay constant (1/s). 4. Activity A of a radioactive source at a time t:…arrow_forwardPart A Some atomic masses Particle Symbol Mass (u) Electron e 0.00055 Part B Proton 1.00728 Neutron 1.00866 Part C Hydrogen 1.00783 Helium 4He 4.00260 Part D Part E Part F Calculate the binding energy per nucleon of the helium nucleus He. Express your answer in megaelectron volts to two significant figures. ? MeVarrow_forward3C. Write the equation for the beta-positive decay of Potassium-40. b) Write the element that is formed in the beta-positive decay of Potassium- 40. Your answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios