
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
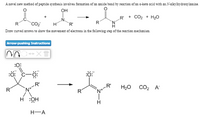
Transcribed Image Text:A novel new method of peptide synthesis involves formation of an amide bond by reaction of an a-keto acid with an N-alkylhydroxylamine.
OH
+ CO2 + H20
CO2
TR'
Draw curved arrows to show the movement of electrons in the following step of the reaction mechanism
Arrow-pushing Instructions
:0:
:ö: c-ö:
-R'
.R'
H20 CO2 A
R
R
H-A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How is THC-O acetate synthesized from delta-9 THC? Please draw out the reaction mechanism and cite your sources with an explanation for each step.arrow_forwardDetermine the product of the following sequence:arrow_forwardGve the organic product(s) formed at the end of the following sequence or reactions: ia Benzene I III Cl Zn(Hg) + HCl(aq) Ch light AICI (catalyst) Heat E and Z isomers II IV OH (aq) E2 elimination OHarrow_forward
- The IUPAC name for the compound shown is CH₂-CH CH3-CH2-C-NH-CH3 NH CHO O N-methylpropionamide ON-methylpropanamide O 1-methylpropanamide O 2-butanamidearrow_forwardCarbonic anhydrase is an enzyme that catalyzes the conversion of carbon dioxide to bicarbonate ion. It is a metalloenzyme, with Zn2+ coordinated at the active site by three histidine side chains. Propose a mechanism for the reaction.arrow_forwardPlease don't provide handwriting solutionarrow_forward
- כיו הרוesereםס Arrange the following compounds in the decreasing order of pKa values. OH OH OH CH;CH,CH,OH CH3-CH-CH2OH ČI ČH3 ÑO2 1 4 5 2 A 4,5,3,2,1 B 1,2,4,5,3 1,2,3,5,4 1,2,3,4,5arrow_forwardMetabolism of arginine produces urea and the rare amino acid ornithine. Ornithine has an isoelectric point close to 10.Propose a structure for ornithinearrow_forwardIdentify the product in the following reaction. OH je je A) I CI 22 B) II OH pyridine C) III IV OHarrow_forward
- 5. One method to make N-glycosidic bond for uridine synthesis is the reaction between 1-amino-2,3,5-tri-O-benzoyl- D-ribose and B-ethoxy-N-ethoxycarbonylacrylamide. Propose a likely mechanism for the reaction. Bz0. NH₂ OBz OBz + Eto Eto ΝΗ O Bz0. OBz OBz ΝΗ OH™ H₂O uridinearrow_forwardMeO + [0] DME heat Hint: C₁1 H100 O Hint: One of the byproducts formedarrow_forwardCF 3- Celecoxib -SO2NH2 Do the following synthesis reactions. ŞO₂NH₂ NHNH2 A +arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY