
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
A new type of galvanic fuel cell is being developed which uses the following half- reactions:
- I included a picture that shows the half-reactions to use for the questions.
A) What is the standard cell potential for this cell? 
B) What is the overall reaction for this cell? 
C) How much work can this cell do under standard conditions?
D) If a cell is charged with 1 atm H2, 1 atm N2, 0.400 M N2H4, 0.00100 M OH- in 5.0 L of water, then what is the cell potential for this cell? Will it be spontaneous under these conditions? 
E) What would be the effect on the cell potential if the pH of this system were decreased?
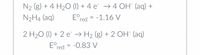
Transcribed Image Text:N2 (g) + 4 H2O (1) + 4 e¯ → 4 OH" (aq) +
N2H4 (aq)
E°red = -1.16 V
2 H20 (1) + 2 e → H2 (g) + 2 OH" (aq)
E°red = -0.83 V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please explain and give the correct answerarrow_forward3. Consider the HCN-KCN buffer system, which species would react if a dilute aqueous HBr solution were added to the buffer? O CN O CN O HCN O KCNarrow_forward1. Draw a diagram that shows a battery made of three galvanic cells. How does the voltage of the battery compare with the voltage of its cells? | ©arrow_forward
- O ELECTROCHEMISTRY Designing a galvanic cell from tw... A chemist designs a galvanic cell that uses these two half-reactions: standard reduction half-reaction 2H₂O(1)+2e Write a balanced equation for the half-reaction that happens at the anode. Ered= Br₂(1)+2e Ered = +1.065 V Answer the following questions about this cell. Write a balanced equation for the half-reaction that happens at the cathode. Write a balanced equation for the overall reaction. that powers the cell. Be sure the reaction is spontaneous as written. Do you have enough information to calculate the cell voltage under standard conditions? If you said it was possible to calculate the cell voltage, do so and enter your answer here. Round your answer to 3 significant digits. 0 0 0 O Yes O No H₂(g) + 2OH(aq) 2 Br (aq) V potential = -0.83 V - 3 ⠀ ローロ X 2 5 x10 Sarrow_forwardUse the given galvanic cell notation below to answer the following questions. 2+ Sn (s) | Sn²+ (aq) || Ti+ (aq)| Ti (s) a) Write the oxidation half reaction. Balance and include states. b) Write the reduction half reaction. Balance and include states. c) Write the overall cell reaction. Balance and include states. d) Identify the anode and cathode. Label your answers a), b), c), d).arrow_forwardCannot Figure out either of the questionsarrow_forward
- Which of the following statements is(are) true for all galvanic cells? 1. Reduction occurs at the cathode. II. The anode gains mass during discharge (note: this means operation of the cell.) III. The voltage is less than or equal to zero. O only I O only III O only II O L I, and II O Il and IIarrow_forwardHow do you determine which half-reaction is at the cathode and which at the anode in a voltaic cell?arrow_forwardDesign an experiment to measure the standard potential of a Silver Describe your electrochemical cell in detail, including the setup, the chemical reactions and notation. \Write up your experiment in such a way that a chemist can carry out your instructions).arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY