
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Bb.45.
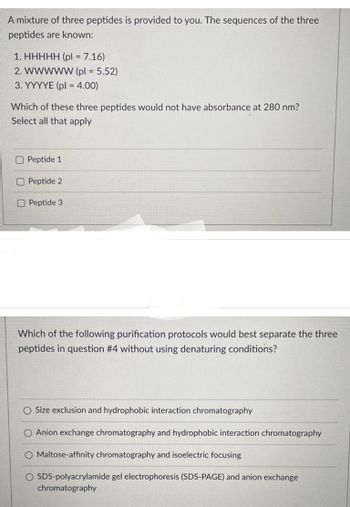
Transcribed Image Text:A mixture of three peptides is provided to you. The sequences of the three
peptides are known:
1. HHHHH (pl = 7.16)
2. WWWWW (pl = 5.52)
3. YYYYE (pl = 4.00)
Which of these three peptides would not have absorbance at 280 nm?
Select all that apply
Peptide 1
Peptide 2
O Peptide 3
Which of the following purification protocols would best separate the three
peptides in question #4 without using denaturing conditions?
O Size exclusion and hydrophobic interaction chromatography
O Anion exchange chromatography and hydrophobic interaction chromatography
O Maltose-affinity chromatography and isoelectric focusing
O SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and anion exchange
chromatography
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- 1. Draw the appropriate titration curve for the tripeptide Met-Lys-Val starting at pH 1 and ending at pH 12. Label the pKas and the pI. Draw the two different forms of the molecule that is present at each buffering region. 2. Draw the structure of the peptide Arg-Met-His-Val-Glu and circle the coplanar atoms in one peptide bond. 3. Determine the pI for the peptide given in question 2 above.arrow_forwardIdentify and briefly describe three different PEGylation strategies you can use to PEGylate this peptide and draw the chemical structure of the resulting mPEG peptide conjugatesarrow_forwardWhich of the peptide sequences below best matches the hydropathy plot shown? L 10 Residue Number 4 2 Hydropathy value -2 0 セー RAFLILFMTYFLILFLI ILYYAGSREDHSGYLIL OLHGDQNRERDGHSQERD EQSDTERNQHGALIYLI 01 5 15arrow_forward
- Please don't provide handwriting solutionarrow_forwardThe globular proteins tend to have a high-dimensional folding in their higher-order structure g providing very limited analysis when conducting peptide analysis. Explain what steps would be suitable to prepare a successful chromatographic peptide mapping?arrow_forwardCan you please help find the ph 9.5 of ser?arrow_forward
- please help in under 30 minsarrow_forwardPlease don't provid3e handwritten solution ....arrow_forwardcalculate the volume of stock solutions required to make up the buffer solutions that will be used for protein purification. The solutions you need to prepare for purification are: i. Binding Solution A: make up 50 mL 50 mM HEPES buffer (pH 7.5), 300 mM NaCl, 5mM imidazole, 5% (v/v) glycerol ii. Wash Solution B: make up 50 mL 50 mM HEPES buffer (pH 7.5), 300 mM NaCl, 75mM imidazole, 5% (v/v) glycerol iii. Elution Solution C: make up 10 mL 50 mM HEPES buffer (pH 7.5), 300 mM NaCl, 500 mM imidazole, 5% (v/v) glycerol please show your working . Thnk youarrow_forward
- You are given an equimolar (0.10 mM) mixture of Ubiquitin protein that is free/pure of any other macromolecules (e.g. nucleic acids) in a pH 7.0 phosphate buffer How can you purify Ubiquitin? Propose a 2-step minimum plan to purify their protein. Basic rules: Affinity chromatography (e.g. His Tag purification) is not an allowed step because these proteins are in their native state (i.e. – do not have a polyhistidine tag). Also, you are not allowed to rely solely on the color of certain proteins (e.g. cytochrome C and GFP) in your characterization/proposal. You are allowed to buffer exchange (i.e. – switch buffers) but try to keep your pH’s in a reasonable range (5.5 – 8.5) or you risk denaturing your protein! Assume you can only separate proteins under 50.0 kDa with a 5.0 kDa difference using SEC. Otherwise, assume proteins greater than 50.0 kDa are unable to be separated. Assume proteins with the same charge (positive or negative) are not easily separated using IEC. Assume all…arrow_forwardWhich of the peptide sequences below best matches the hydropathy plot shown? 1. ILYYAGSREDHSGYLIL 2. RAFLILFMTYFLILFLI 3. LHGDQNRERDGHSQERD 4. LIFLAIFPAGSTSEDRR 5. EQSDTERNQHGALIYLIarrow_forwardHelp mearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON