
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
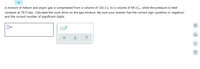
Transcribed Image Text:A mixture of helium and argon gas is compressed from a volume of 100.0 L to a volume of 99.0 L, while the pressure is held
constant at 39.0 atm. Calculate the work done on the gas mixture. Be sure your answer has the correct sign (positive or negative)
and the correct number of significant digits.
画
x10
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 2.63 g sample of a substance suspected of being pure gold is warmed to 72.8 degrees Celsius and submerged into 15.5 g of water initially at 24.5 degrees Celsius. The final temperature of the mixture is 27.0 degrees Celsius. What is the specific heat capacity of the unknown substance? Express your answer using to significant figures.arrow_forwardA piece of unknown metal with a mass of 23.78 g is heated in boiling water to 97.4°C and then dropped into a coffee cup calorimeter containing 72.71 g of water at 13.65°C. When thermal equilibrium is reached, the final temperature is 24.79°C. Calculate the specific heat capacity of the unknown metal. The specific heat of water is 4.184 J/g/°C. Round your answer to 2 decimal places.arrow_forwardA chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 9.80kg of water at 27.4°C . During the reaction 140.kJ of heat flows out of the flask and into the bath. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18J·g−1·K−1 . Be sure your answer has the correct number of significant digits.arrow_forward
- A calorimetry experiment was done on a mystery piece of metal and the data was recorded in the table below. Measurement 89.20 g Mass of the metal Initial temperature of the metal Mass of the water Initial temperature of the water Final temperature of the water 98.1 °C 47.50 g 22.5 °C 28.2 °C What was the specific heat of the mystery metal? (CH20 = 4.181 J/gºC ) Round your answer to 2 decimal places.arrow_forwardbe sure your answer is correct and have the correct number of significant digitsarrow_forwardCalculate the energy required to heat 403.0 mg of water from 36.8 °C to 57.0 °C. Assume the specific heat capacity of water under these conditions is 4.18 J-g .K . Be sure your answer has the correct number of significant digits. 0 0x10 ロ・ロ X μ 00 4 Sarrow_forward
- Calculate the energy required to heat 718.0 mg of water from 46.0 °C to 56.7 °C. Assume the specific heat capacity of water under these conditions is -1 -1 4.18 J∙g¯¹·K-¹ Be sure your answer has the correct number of significant digits. 0 x10 X μ 010 ? 00. 18 Ararrow_forwardA mixture of helium and hydrogen gas is compressed from a volume of 81.0 L to a volume of 70.0 L, while the pressure is held constant at 34.0 atm. Calculate the work done on the gas mixture. Be sure your answer has the correct sign (positive or negative) and the correct number of significant digits.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY