
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
the answer is correct I just need the calculations please
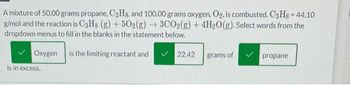
Transcribed Image Text:A mixture of 50.00 grams propane, C3Hs, and 100.00 grams oxygen, O₂, is combusted. C3H8 - 44.10
g/mol and the reaction is C3H8 (g) +50O2(g) → 3CO₂(g) + 4H₂O(g). Select words from the
dropdown menus to fill in the blanks in the statement below.
is the limiting reactant and
Oxygen
is in excess.
✓ 22.42
grams of
propane
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write each of the following numbers in exponential or scientific notation. Write each number to two decimal places with only one non-zero digit before the decimal point. Example: Use not or . 7420 0.000989 56900 0.00342 Submit Answerarrow_forwardReduce the following terms to right number of Significant Figures. to 3 s. f. to 2 s. f. to 1 s. f. 6.3528 34.026 0.005708 150.932 0.00007835 Sorry i am having difficulty making a columnsarrow_forwardHow to determine the number of significant figures when computing percent error? Is it based on the experimental value or theoretical value? or both?arrow_forward
- The first instrument we will use in lab is called a graduated cylinder. A graduated cylinder reads starting from zero at the bottom and goes up as liquid fills it. Keep in mind that you should always estimate one digit further than what is marked on the device to preserve your significant figures. For example, if the device has 1.0 mL increments, the measurement can be estimated to the second decimal place. For the graduated cylinder below, what would be the correct volume reading considering that the markings are in milliliters? Hint: What are the increments of measurement on the graduate cylinder below? -60 -50 -40 -30 -20arrow_forward14. A student determined the density of air (STP conditions) to be 1.19 grams/Litre. If the theoretical value is 1.29 grams/Litre, determine the student's percentage error.arrow_forwardneed help with this chemistryarrow_forward
- A runner wants to run 3.9 miles. She knows her running pace is 4.5 km/hr. How many minutes will it take her? USE DIMENSIONAL ANALYSISarrow_forwardI need help with how many miles there must have been in the sample?arrow_forwardMacmillan Learning Red gold is a gold-copper alloy used to make jewelry. A piece of jewelry made of red gold weighs 8.50 g and has a volume of 0.535 cm. Gold has a density of 19.3 g/cm² and copper has a density of 8.96 g/cm'. Calculate the percentage by mass of each metal in the jewelry. Assume the total volume of the jewelry is the sum of the volumes of the two metals it contains. gold: copper. Pure gold is defined as having 24 carats. When mixed in an alloy, the carats of gold are given as a percentage of this value. For example, a piece of jewelry made with 50% gold has 12 carats. State the purity of this piece of red gold jewelry in carats. purity: caratsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY