
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
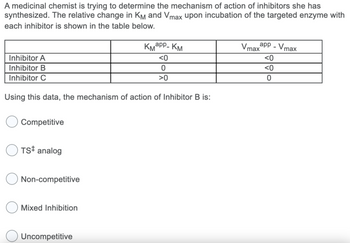
Transcribed Image Text:A medicinal chemist is trying to determine the mechanism of action of inhibitors she has
synthesized. The relative change in KM and Vmax upon incubation of the targeted enzyme with
each inhibitor is shown in the table below.
Inhibitor A
Inhibitor B
Inhibitor C
Using this data, the mechanism of action of Inhibitor B is:
Competitive
OTS‡ analog
Non-competitive
Mixed Inhibition
КМарр- КМ
<0
0
>0
Uncompetitive
app - Vmax
<0
<0
0
Vmax

Transcribed Image Text:Metalloproteases can contain a catalytic triad made up of:
O D(H₂O)D
O E(Ln*)S
O None of the choices are correct
O EKS
ODHS
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Some Protein P binds to 1 molecule of X. The fractional binding curve (θ vs. [Ligand]) shows that θ = ⅓ when [X] = 6 mM. What is the Kd for X binding to P?arrow_forwardWhat is the relative activite and the degree of inhibition caused by a competitive inhibitor when [S] = Km and [I]=ki?arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- Examine this depiction of the initial steps of catalysis for a proteolytic enzyme. Oxyanion hole 93 with In 1-2 sentences, describe the role being played by the amino acid hydroxyl side chain in the step labeled 2. In 1-2 sentences, describe the role of the oxyanion hole. In 1-2 sentences, describe the role of the His is the step labeled 3.arrow_forward29. In chymotrypsin both histidine and serine are important amino acids in the catalytic reaction. Below is an image showing one step of the reaction. Describe what is happening and the roles histidine and serine are playing. In your answer be sure to use correct terms in describing the type of enzyme mechanism being done each amino acid. His Asp 102 H₂C CH₂ Ser (195) CH₂ 4 Asp 102 H₂C CH₂ His 57 New N-terminus of cleaved polypeptide R chain Ser 195 CH₂arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- An enzyme catalyzes the reaction M ⇌ N. The enzyme is present at a concentration of 1 nM, and the Vmax is 2 μM s−1. The Km for substrate M is 4 μM. (a) Calculate kcat. (b) What values of Vmax and Km would be observedin the presence of sufficient amounts of an uncompetitive inhibitor to generate an α′ of 2.0?arrow_forwardTrypsin is a serine protease that has a KM of 15 mM. At an initial substrate concentration of 250 µM insulin, trypsin is found to degrade insulin at a rate of 0.6 µM/min. If the concentration of trypsin in solution was 4.326 µM, what is the kcat of trypsin in s-1?arrow_forwardQuestion #44arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY