
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:A local AM radio station broadcasts at a frequency of 817 kHz. (1 kHz = 1000 s¹)
Calculate the energy of the frequency at which it is broadcasting.
Energy =
kJ/photon
Submit Answer
Use the References to access important values if needed for this question.
Try Another Version
1 item attempt remaining
Expert Solution
arrow_forward
Step 1
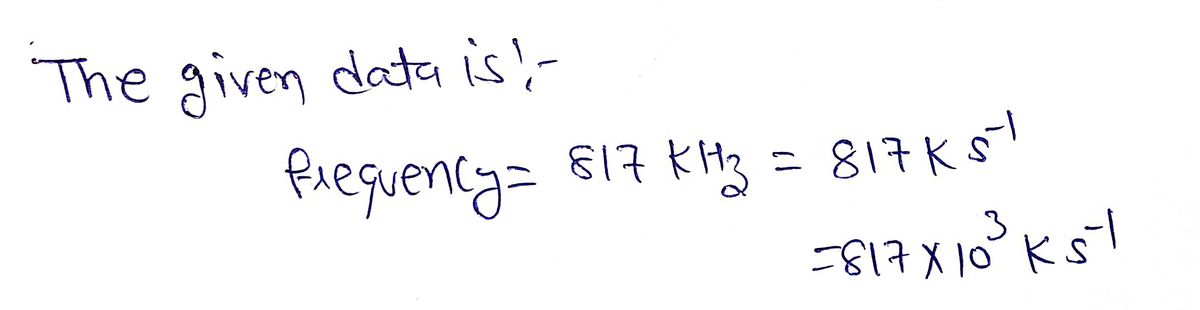
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Check my answer, I am afraid I skipped something in question 10. What is the above FREQUENCY/WAVELENGTH for one photon and one mole?arrow_forwardI see the same question is answered but the part where it says " That is, Equation 2" I am still confused by the answers the other questions gave. I would like more explanationarrow_forwardA local FM radio station broadcasts at an energy of 5.98×10-29 kJ/photon.Calculate the frequency at which it is broadcasting.Frequency = MHz(1 MHz = 106 sec -1)arrow_forward
- [R Use the References to access impo A local FM radio station broadcasts at a frequency of 88.2 MHz. Calculate the energy of the frequency at which it is broadcasting. Energy = KJ/photon (1 MHz = 106 sec 1) Submit Answer Try Another Version 2 item attempts remainingarrow_forwardA for one line of the hydrogen spectrum is .4118 x 104 cm. Use this value in the Rydberg equation to calculate the RH value using n1 2, and n2 = 4. 3.477 Submit Answer Incorrect. Tries 3/99 Previous Tries This discussion is closed.arrow_forwardAccording to the Experiment #8 PowerPoint provided in the Experiment #8 module on your lab section's Canvas page, if the emission spectrum signal is weak once everything is setup and on for Part B of the experiment (collecting data from the Hydrogen Emission Spectrum), who should modify the position of the wand? only my lab instructor only myself there will not be a need to adjust it at any point only my lab partner or someone in my lab grouparrow_forward
- im stuck on question 1arrow_forwardUse the References to access important values if needed for this question. A local FM radio station broadcasts at a frequency of 94.1 MHz. Calculate the wavelength at which it is broadcasting. Wavelength meter (1 MHz = 10° s -1) Submit Answer Try Another Version 2 item attempts remaining t otarrow_forwardPlease try to answer itarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY