
A liquid phase reaction takes place in a semi-batch reactor. The reaction that occurs is a first order reaction which is given as follows:
A → B , r = kCA
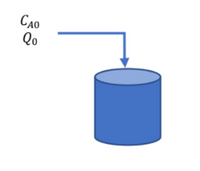
where r (mol/L∙sec) is the reaction rate, CA (mol/L) is the concentration of reactants in the reactor, and k (1/sec) is the kinetic constant of the reaction. Reactor tank having tank volume VR and at the beginning the tank is empty. At time t = 0, solution A with a concentration of CA0 is fed into the reactor at a constant rate, namely Q0. In the feed stream, the concentration of B = 0 (CB0 = 0). Assumption: the density of the liquid does not change. If you know the volume of liquid in the tank is V, and the moles of A in the tank, nA, then construct a differential equation, then determine the correct solution method and solve the differential equation.
Remember that CA = nA/V
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 1 images

- complete part E onlyarrow_forwardPowerplant cooling water with a temperature of 35 °C and a flowrate of 10 m3/s is discharged into a river with a temperature of 10 °C and a flowrate of 50 m3/s.a) What will the new temperature of the river be downstream of the mixing point? Draw and label your figure and state any assumptions.b) Using your knowledge of Henry’s law to justify your answer, how will this change in river temperature influence the amount of oxygen gas dissolved in the water?arrow_forwardIn a batch reactor, a substance A was processed, which generated different products (D and U), through competitive parallel reactions with the following reaction kinetics: After 20 minutes of reaction, it was determined that the composition of the reaction medium was CA = 1 mol/L, CD = 5 mol/L, CU = 2 mol/L. The option that indicates, respectively, the instantaneous and global selectivities at the end of the reaction are: A) 3 and 2. B) 2.5 and 2. C) 2 and 3. D) 2 and 2.5.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





