
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
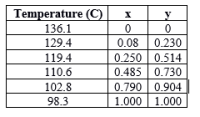
A liquid mixture containing 10 mol% heptane and 90 mol% ethylbenzene is fed at its boiling point to the top of the stripping tower at 101.32 kPa abs. The bottoms are to contain 99% ethylbenzene. For every 5 mol of feed, 3 mol of vapor is withdrawn as product. Calculate the composition of the vapor and the number of theoretical plates required. The equilibrium data below are given as mole fraction heptane.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- The lower heating value for gaseous n-octane is 44,791 kJ/kg, and its latent heat of vaporization is 300 kJ/kg. Using this information, determine the enthalpy of formation at 298 K for liquid n-octane in kJ/kmol. (Here you are to compute the enthalpy of formation from the given information, not look it up in a property table.)arrow_forwardA fully charged battery contains 0.02 kmol Ag₂O (silver oxide) and 0.02 kmol Zn (zinc). The battery is initially at a temperature of 21°C. The fully charged battery is then discharged for half an hour under adiabatic conditions (no heat leaves the system). During the half hour of being discharged, the electric current from the battery does 200 kJ of work external to the battery system (none of this external work heats the battery). During this half hour, 0.001 kmol of both reactants are consumed in the net cell discharge reaction: Please see the following table for heat capacities and heats of formation. The heats of formation below show the amount of energy (kJ) needed to form one kmole of each component. If the heat of formation is negative, then forming that species releases energy (to do work or provide heat). Therefore, to create one kmole of silver oxide 29000 kJ energy is released and is generated in the battery (for work or heat), and if one kmole of silver oxide decomposes…arrow_forwardCalculate the heat capacity for formaldehyde at 25°C. Answer should be in kJ/kgmol°Carrow_forward
- Insulated Sample Burning outside dish sample chamber Steel bomb Combustion (bomb) calorimeter. In an experiment, a 0.6629 g sample of phthalic acid ( C8H604) is burned completely in a bomb calorimeter. The calorimeter is surrounded by 1.046 x 10³ g of water. During the combustion the temperature increases from 24.04 to 26.63 °C. The heat capacity of water is 4.184 J-g¹. C-¹. The heat capacity of the calorimeter was determined in a previous experiment to be 788.9 J. ˚C-¹. Assuming that no energy is lost to the surroundings, calculate the molar heat of combustion of phthalic acid based on these data. C8 H6 O4(s) + (15/2) O2(g) → 3H₂O(l) + 8CO2 (g) + Energy kJ/mol Molar Heat of Combustion =arrow_forward6. A 15.0 gram sample of silver metal is heated to 96.7∞C and then dropped into 55.0 grams of water at 23.0∞C. Assuming that all the heat lost by the silver is absorbed by the water, calculate the final temperature of the silver and waterarrow_forwardA countercurrent tunnel dryer is used to dry apple slices from an initial moisture content of 65% to 5% (wet basis). Hot air enters at 95 ° C with 1% RH, and exits at 52.0 ° C. If the product temperature along the dryer is 20 ° C and the solid specific heat of the product is 2.2 kJ / (kg ° C), determine the rate of hot air required to dry the wet product at a rate of 200 kg / hr. Determine the RH of the dryer exhaust air. a. Drying air rate (ma) = kg / hr. b. RH of dryer exhaust air =%arrow_forward
- Condensation of Steam on Vertical Tubes. Steam at 1 atm pressure abs and 100°C is condensing on a bank of five vertical tubes each 0.305 m high and having an OD of 25.4 mm. The tubes are arranged in a bundle spaced far enough apart so that they do not interfere with each other. The surface temper- ature of the tubes is 97.78°C. Calculate the average heat-transfer coefficient and the total kg condensate per hour. Ans. h = 15 240 W/m².Karrow_forwardQ3(a) A stream of benzene vapor at 580 °C and 1 atm is cooled and converted to a liquid at 25 °C and latm in a continuous condenser. (i) (ii) Illustrate a completely process path for the above process. Calculate the total enthalpy change for this process.arrow_forward8.29 Show complete solution and diagramarrow_forward
- The results of air temperature measurement obtained a dry bulb temperature of 37 ° C and a wet bulb temperature of 27.5 ° C. Using a psychrometric chart, determine the properties of the air as follows:a. RH:%b. Water content: kg water / kg airc. Specific volume: m3 / kgd. Enthalpy: kJ / kge. Condensation temperature: ° CIf the air is within 162 m3 space, determinef. Air weight (dry air and water vapor): kgg. the amount of water content in the space: kgarrow_forwardThe standard enthalpy of Ethanol (C2H5OH) is -278 kJ.mol. Find the gross heat released when 100 kg is burned.arrow_forward9.12 A 50 wt% Ni-50 wt% Cu alloy is slowly cooled from 1400°C (2550 F) to 1200°C (2190 F). (a) At what temperature does the first solid phase form? (b) What is the composition of this solid phase? (c) At what temperature does the liquid solidify? (d) What is the composition of this last remaining liquid phase?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The