
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A Lewis structure for OF2, H3COH and NO+ are shown below. Pick the best answer. (Note: formal charges are circled.)
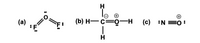
Transcribed Image Text:(а)
(b) н— с—о
—н (с) :N
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the following Lewis structure of [SiF6]²;, every atom, bond and lone pair is positioned. To complete the structure, drag the formal charge tags to the appropriate atom(s). Each marker may be used more than once, or not at all. If an atom has a formal charge of zero, do not drag a tag to it. When you drag the marker in, place the little crosshairs in the upper left corner of the marker directly over the atom(s) in question (not above them). :F: :F: 2+ 2- :L: :L:arrow_forwardThe structure of nitric acid (HNO3), shown below, is incorrect. Which of the following statements, or combin statements, best describes the reason? The structure should have a +1 formal charge. oo many valence electrons in the molecule. e central Natom violates the octet rule. central N atom does not have a lone pair. HO :0=2arrow_forwardWrite the Lewis structures for CH2N2, including all resonance forms, and show formal charges.arrow_forward
- Draw Lewis structure(s) for the carbonate lon (CO₂). If there are equivalent resonance structures, draw all of them. n D co₂2: 0 . Draw one structure per sketcher box, and separate added sketcher boxes with the symbol. Do not include overall lon charges or formal charges in your drawing. Do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. ● 6 # H Ⓒ CH, CHO: 0 Y Chemic b Draw Lewis structure(s) for the acetaldehyde molecule (CH₂CHO). If there are equivalent resonance structures, draw all of the POLICE 81 MEDITE HARA (4) Y Draw one structure per sketcher box, and separate added sketcher boxes with the symbol. Do not include overall ion charges or formal charges in your drawing. Do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. ARQQA 000-ZIF www HEADING Chartlkoodn MES DE A V Ja remove 000-n [ MacBook Airarrow_forwardPlease don't provide handwritten solution.arrow_forward1. Write Lewis symbols for the following atoms. (1pt each) (a) Kr (b) Ge (c) N (d) Ga (e) As (f) Rb 2. Write plausible Lewis structures for the following molecules that contain only single covalent bonds. (2 pts each) (а) FCI (b) I2 (c) SF2 (d) NF3 (е) Н-Те 3. By means of Lewis structures, represent bonding between the following pairs of elements (Your structures should show whether the bonding is essentially ionic or covalent): (2 pts each) (a) Cs and Br (b) H and Sb (c) B and Cl (d) Cs and Cl (e) Li and O (f) Cl and I 4. Assign formal charges to each of the atoms in the following structures. (3 pts each) (a) [H–C=C:]¯ (c) [CH3–CH-CH3]* (b) |2– :0: :0: 5. What is the formal charge of the indicated atom in each of the following structures? (2 pts each) (a) the central O atom in 03 (b) Al in AIH4- (c) Cl in Cl03 (d) Si in SiF62- (e) Cl in CIF3 6. Arrange the following elements in the order of decreasing electronegativity: fluorine, bromine, lithium, francium, silicon. (1 pt each per…arrow_forward
- In the BEST Lewis structure for hydrogen cyanide, HCN, the formal charge charge on H is __________, the formal charge on C is___________ , and the formal charge on N is _________. Indicate formal charge with a sign followed by a numerical charge (e.g. -2, +1) unless the charge is 0, then enter 0.arrow_forward1. a) draw the complete Lewis structure (atoms, dots, lines, dashes, wedges, formal charges) for the formula SF4 b)draw the complete Lewis structure (atoms, dots, lines, dashes, wedges, formal charges) for the formula NH4+arrow_forwarda. Write a Lewis structure that obeys the octet rule for the following species. Assign the formal charge for the central atom of XeO4. If multiple resonance structures exist, use one that does not involve an expanded valence. Formal charge: b. Write a Lewis structure that obeys the octet rule for the following species. Assign the formal charge for the central atom of clO3¯. If multiple resonance structures exist, use one that does not involve an expanded valence. Formal charge:arrow_forward
- Consider the following ion: BrO3−. a) Show the full electron configuration for Br. b) Draw the most correct Lewis structure for BrO3− and briefly explain why your Lewis structure is correct. c) If the structure is stabilised by resonance, draw at least one of the possible resonance forms. If it is not stabilised by resonance, briefly explain why. d) What is the electronic geometry of BrO3−? What is its molecular shape? e) Does BrO3− have a dipole moment? Briefly justify your answer. f) On average, would you expect IO3− to have longer or shorter bonds than BrO3−? Briefly explain your answer. g) Which of the following molecules would you expect to have the lowest vapour pressure? Briefly explain your choice. (IMAGE WITH POSSIBILITIES) h) What is the molecular formula for Compound C? What is the empirical formula for Compound C?arrow_forwardThe value of Ka1 of hypophosphorous acid, H3PO2, is nearly the same as the Ka1 of phosphoric acid, H3PO4. Draw a Lewis structure for phosphoric acid that is consistent with this behavior. Draw the Lewis structure with the formal charges minimized. Do not include formal charges in your structure. Include hydrogen atoms and nonbonding electrons in the structure.arrow_forwardI need help on 4barrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY