
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Can you give me the solution for this one please?
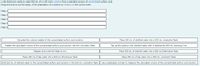
Transcribed Image Text:A lab technician wants to make 500 mL of a 2.50 mol/L solution from a standard solution of concentrated sulfuric acid.
Drag and drop to put the steps, of the preparation of a solution by dilution, in the correct order.
Step 1
Step 2
Step 3
Step 4
Step 5
Calculate the volume needed of the concentrated sulfuric acid solution.
Place 250 mL of distilled water into a 500 mL volumetric flask.
Pipette the calculated volume of the concentrated sulfuric acid solution into the volumetric flask.
Top up the solution with distilled water until it reaches the 500 mL meniscus line.
Stopper and invert the flask to mix.
Place 250 mL of distilled water into a 500 mL Erlenmeyer flask.
Place 250 mL of tap water into a 500 mL Erlenmeyer flask.
Place 250 ml of tap water into a 500 ml volumetric flask.
Add 250 mL of distilled water to the concentrated sulfuric acid solution in the 500 mL volumetric flask.
Use a graduated cylinder to measure the calculated volume of the concentrated sulfuric acid solution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me, double check your answers as previous tutors got it wrong.arrow_forwardIf I have 340 mL of a 0.500 M NaBr solution, what will the concentration be if I add 560 mL more water to it? a. 0.304 M C. 0.189 M b. 0.311 M d. 0.824 Marrow_forward6 A compound of iodine and cesium contains 74.85 g of metal and 61.30 g of nonmetal. How many grams of cesium are in 34.88 g of compound?arrow_forward
- given a diluted acid solution that was tested by another student. They were supposed to make a solution with a certain concentration. If your experimental data matched the test but not the target concentration, what does that tell you about the data? For example, the student was supposed to make a 0.87 M solution. When they tested it, they got 0.6113 M. When we tested it,we got 0.6144 M.arrow_forwardΠ U U Oxalic acid (H2C2O4) is a polyprotic acid. Write balanced chemical equations for the sequence of reactions that oxalic acid can undergo when it's dissolved in water. ローロarrow_forwardThe concentration of sodium sulfate solution is 19.1% by mass. There are ______ g of sodium sulfate present in a 277.9g sample of this solution. A. 6.87×10−4 B. 1450 C. 5310 D. 53.1 E. 6.87arrow_forward
- To do a chemistry experiment, you need to prepare 350mL of 0.25M NaOH. There is one small problem: you do not have any 0.25M NaOH. However, all is not lost, it has been discovered that you do have a stock supply of 6M NaOH in your cupboard. How do you prepare the solution you need? (Write a recipe for making the solution as the final step. )arrow_forwardThe acids and bases used in the first part of this experiment were strong acids and strong bases. What is a weak acid or base? simple plzarrow_forwardAn acid will always neutralize a base to form a salt and water. True or False?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY