
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
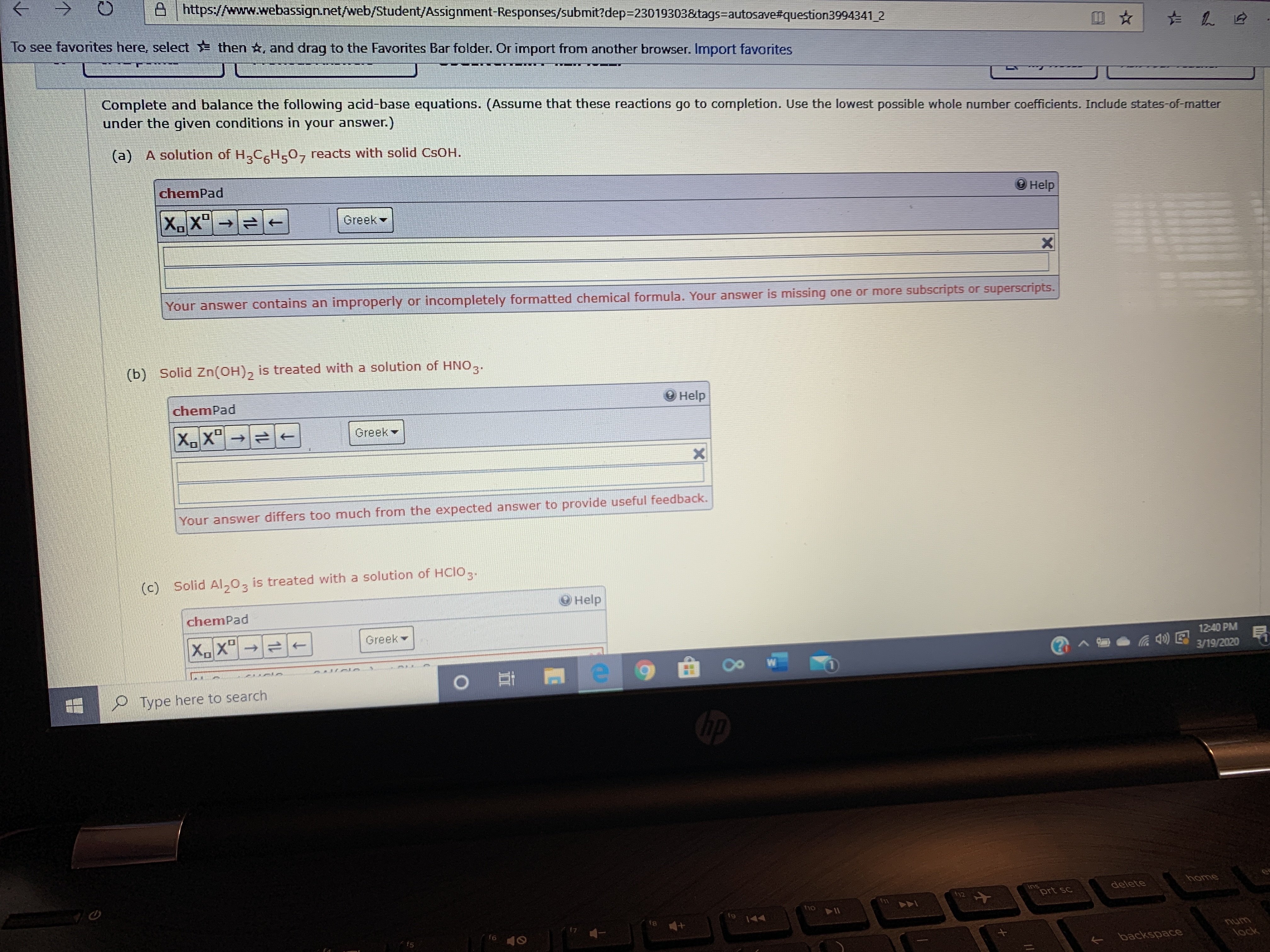
Transcribed Image Text:A https://www.webassign.net/web/Student/Assignment-Responses/submit?dep=23019303&tags=autosave#question3994341 2
To see favorites here, select then *, and drag to the Favorites Bar folder. Or import from another browser. Import favorites
Complete and balance the following acid-base equations. (Assume that these reactions go to completion. Use the lowest possible whole number coefficients. Include states-of-matter
under the given conditions in your answer.)
(a) A solution of H,CH,0, reacts with solid CSOH.
chemPad
О нelp
X.X"
to
Greek -
Your answer contains an improperly or incompletely formatted chemical formula. Your answer is missing one or more subscripts or superscripts.
(b) Solid Zn(OH), is treated with a solution of HNO,.
chemPad
Help
XX"→
Greek
Your answer differs too much from the expected answer to provide useful feedback.
(c) Solid Al,0,
is treated with a solution of HCIO,.
O Help
chemPad
Greek
12:40 PM
? ^
3/19/2020
10
Type here to search
home
ins
prt sc
delete
t12
f11
fho
f9
f8
144
unu
lock
f6
f5
E backspace
1L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- When the following skeletal equation is balanced under acidic conditions, what are the coefficients of the species shown? _____SO42- +______ NO------> _____H2SO3 + ____ HNO3Water appears in the balanced equation as a_______ (reactant, product, neither) with a coefficient of _______. (Enter 0 for neither.) Which species is the oxidizing agent?arrow_forwardProvide an example of an everyday (i.e. in your own life) use/implication of a) synthesisreaction, b) combustion.arrow_forwardPlease help answer this question 29 write out each of the reactions found when forming an aqueous solution of selenious acid (H2Se04 (aq), a polyprotic weak acid). Hint: there should be 3 reactions to write out for this solution, and they can be written in the boxes below in any order.arrow_forward
- + ■ 3 * 8 W Chapter... G N. 3 ebpage is using significant memory. Closing it may improve the responsiveness of your Mac. = Supporting Materials Periodic Table Additional Materials eBook HK Submit Answer webassign.net Constants and Factors The hydronium ion concentration in a sample of springwater is found to be 7.1x107 M at 15°C. What is the concentration (in M) of hydroxide ions in the springwater? (Kw = 4.57 x 10-15 at this temperature.) M O Supplemental Data E L C a @ + ▸arrow_forwardHello, can you help me with those hmw questions listed here? I struggled with all of those ones and I just do not know my chemical balance equations and net ionic equations am getting an incorrect answer. Can you please help. I did the most of the parts questions can you please check it? I need to write the balanced molecular equations, and then write the balanced net ionic equations for the following reagents that are mixed in aqueous solution or heated. If no reaction occurs, write NR which is no reacton. And thats the directions for this homework. Can you please help me with those ones? I need help with all of them. I really don't know how to do them, but the ones I have attached pics I knew how to do them and notes helped me too. o. Mercury (I) nitrite and iron(III) fluoride . p. Chloric acid and sodium hydroxide. q. calcium chloric potassium carbonate r. potassium hydrogen carbonate and hydrochloric acid s. Acetic acid and calcium carbonate (hint is acetic acid a strong acid?…arrow_forwardHow would you fill this out and answer these questions for Copper penny turning green?arrow_forward
- NET IONIC EQUATIONS: LAB REPORT 1. Be legible! Use an extra sheet of paper if needed. PAY ATTENTION TO PHASE SUBSCRIPTS AND CHARGES! 2. Use a pdf scanning app to convert ALL images of ALL pages into a SINGLE PDF file and save it to a known location on your computer. 3. Submit to LAB REPORT DROPBOX: NET IONIC EQUATIONS BEFORE THE DATE AND TIME LISTED IN THE MASTER CALENDAR OF DUE DATES! For the below two reactions, do the following for each: 1. Write the correct formulas for the products, assuming a metathesis/exchange/double replacement/double displacement reaction is occurring 2. Using a solubility table, predict whether the reaction would produce a precipitate (would proceed spontaneously) and fill in the phase subscripts for each product 3. Balance the equation, giving the correct balanced molecular equation 4. Write the total ionic equation 5. Write the net ionic equation You will need a periodic table, a solubility table, and a table of polyatomic ions to complete this report.…arrow_forwardWhen the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown?_____SO2 + _____ Zn2+------>SO42- + _______ ZnWater appears in the balanced equation as a _______ (reactant, product, neither) with a coefficient of _______. (Enter 0 for neither.)How many electrons are transferred in this reaction? ________arrow_forwardA cvg.cengagenow.com Netf OWLV2 | Online teaching and learning resource from Cengage Learning [Review Topics] [References] Use the References to access important values if needed for this question. Calculate the hydronium ion concentration in an aqueous solution of 0.195 M carbonic acid, H,CO3 (aq). [H,O*]= M. Submit Answer Retry Entire Group 8 more group attempts remaining Visited Email Cengage Learning | Cengage Technical Support MacBook Air 80 888 DD F3 F4 F5 F6 F7 F8 F9 F10 24 % &arrow_forward
- Write the objectives IN YOUR OWN WORDS: Obiective:To predict the products of some displacement reactions and write net ionic equations.Note-the (aq) has been eliminated from all equations below while (s), (I) and (g) have been used to represent phases. When you write chemical reactions in your lab reports youmust show all phases including (aq).arrow_forwardWhen the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown?_____Hg +_____ H3AsO4 -------->______Hg2+ +_____ H3AsO3Water appears in the balanced equation as a ___________ (reactant, product, neither) with a coefficient of __________ . (Enter 0 for neither.)Which element is reduced? ___________________arrow_forwardPlease solve. Show all the work.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY