
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
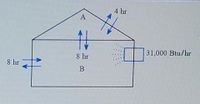
Transcribed Image Text:4 hr
31,000 Btu/hr
8 hr
8 hr
B

Transcribed Image Text:A house, for cooling purposes, consists of two zones: the attic area
zone A and the living area zone B (see below figure). The living area is
cooled by a 2-ton air conditioning unit that removes 31,000 Btu/hr. The
heat capacity of zone B is
8.
°F per thousand Btu. The time constant for
heat transfer between zone A and the outside is 4 hr, between zone B
and the outside is 8 hr, and between the two zones is 8 hr. If the
outside temperature stays at 90°F, how warm does it eventually get in
the attic zone A?
....
It eventually gets to be
(Type an integer or decimal rounded to the nearest hundredth as needed.)
°F in the attic zone A.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- SUPPOSE THE AMBIENT TEMPERATURE IS 20degrees CELSIUS, AND THE HOT RESERVOIR CONSISTS OF A SPHERICAL TANK WITH A RADIUS OF 4.00 m, THAT ACTS AS AN IDEAL EMITTER OF RADIATION. IF ALL THE RADIANT ENERGY EMITTED BY THE TANK COULD BE CAPTURED, WHAT IS THE AVERAGE AMOUNT OF WORK THAT COULD BE DONE EACH SECOND? ( please only answer if your 100% correct) (show work)arrow_forwardAir surrounding a light bulb is moving at 25 m/sec. The surrounding conditions are kept at atmospheric pressure and at temperature 25o C. The light bulb external surface is kept at 140o C. What is the heat transfer rate from the light bulb to the surrounding? Assume the light bulb as a sphere with a diameter of 50 mm.arrow_forwardA radioactive sphere of 20-cm diameter generates heat at the rate of 300 W. It is cooled by placing in a cold inert fluid. The fluid temperature is –20°C. At steady state, the surface temperature of the sphere was found to be 20°C. What is the heat transfer rate from the sphere?arrow_forward
- Q4/ How much heat is required to raise the temperature of 30 Kg air in the room from 5c'to 20c'when a heater is used to heat this room ,under this conditions the specific heat capacity of air=1000 J/Kg.Karrow_forwardSolve it quickly pleasearrow_forwardThermodynamics: Can you show me how to solve for the answer that is written below? Please show it in step by step solution Thank you!arrow_forward
- solve the (1st) question onlyarrow_forwardi want help with all these questions asaparrow_forward6. a. The heat flux applied to the walls of the biomass combustion furnace is 20 W/m2. The furnace walls have a thickness of 10 mm and a thermal conductivity of 12 W/m.K. If the wall surface temperature is measured to be 50oC on the left and 30oC on the right, prove that conduction heat transfer occurs at a steady state!b. Heating the iron cylinder on the bottom side is done by placing the iron on the hotplate. This iron has a length of 20 cm. The surface temperature of the hotplate is set at 300oC while the top side of the iron is in contact with the still outside air. To reach the desired hotplate temperature, it takes 5 minutes. Then it takes 15 minutes to measure the temperature of the upper side of the iron cylinder at 300oC. Show 3 proofs that heat transfer occurs transientlyarrow_forward
- Give an example of application of the Oth law of thermodynamics in everyday life. List explicitly Systems 1, 2, and 3 and what is the purpose of them being at the same temperature.arrow_forwardA house, for cooling purposes, consists of two zones: the atric area zone A and the living area zone B (see below figure). The living area is cooled by a 2 -ton air conditioning unit that removes 23,000 Btu/hr. The heat capacity of zone B is 1/6 °F per thousand Btu. The time constant for heat transfer between zone A and the outside is 3ℎr, between zone B and the outside is 6ℎr, and between the two zones is 6ℎr. If the outside temperature stays at 90°F, how warm does it eventually get in the attic zone A ?arrow_forwardIn an experiment to measure the specific heat of material, a student first measured the masses of the following samples as given in Table 1. Table 1: Measurement of Mass Physical quantity measured in (g) Value Mass of Styrofoam 2.4 cup Mass of Styrofoam 62 cup + water Mass of Brass sample 15.3 Mass of Copper sample 62.5 and then recorded the temperatures of the above two samples, in Table 2. Table 2: Measured values of Temperatures Hot termperature ("C ) Sample Equilibrium cold temperature ( C) temperature ("C) Brass 99.8 26.5 24.5 Сopper 99.8 32 26 Answer the following questions using the data given in Table 1 and Table 2. (Please enter the answers with two digits after decimal) (a) Calculation of specific heat capacity of Brass: Calculated Quantities Mass of the water (in Values grams) Change in temperature lof the hot object ( ATobieca) °C) Change in temperature of water (ATwaer) (°C) Calculated specific heat of the object in (cal/g'C) (b) Calculation of the specific heat capacity…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY