
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Give approximate values for the indicated bond angles in the
following molecules:
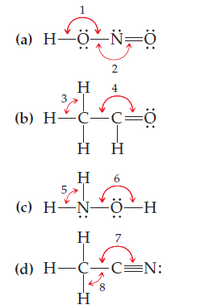
Transcribed Image Text:(a) H~ö-N=Ö
2.
4
(b) H-C-ç=
нн
H
(с) Н—N—Ӧ—н
H
7
(d) H-C c=N:
H
:O:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. Write the Lewis structures for the following substances:BoronPhosphorusBerylliumMagnesium 2. Write the Lewis structures for the following substances:For Carbon monoxideFor carbon tetrachlorideFor a compound formed by oxygen and aluminum atoms.For a compound formed by atoms of calcium and chlorinearrow_forwardConsider the Lewis structure shown below.(a) Does the Lewis structure depict a neutral molecule or anion? If it is an ion, what is the charge on the ion? (b) What hybridizationis exhibited by each of the carbon atoms? (c) Arethere multiple equivalent resonance structures for the species?(d) How many electrons are in the p system of the species?arrow_forwardmog nintmoh noials orlt 2 tedW ol ( bude elval et ainqonqqe toren art werd.V Sologib a sver palom 3. Draw the most appropriate Lewis structure(s) for the ion BH,. What is the electron domain geometry and the molecular geometry? Does the molecule have a dipole?arrow_forward
- Predict the ideal bond angles around each central atom inthis moleculecarbon:: N=C—N—H:NIHnitrogen:НOarrow_forwardMolecule Electron dot structure Valence structure H2Se CH;Brearrow_forwardDraw the products of the following acid/base reactions, depict the proton exchange with electron-pushing arrows, AND circle the proper reaction arrow to indicate if reactants or products (or neither) are favoured at equilibrium. Using the provided pKa table, write all relevant pKa values (i.e. for the two reactants if available and the conjugate acid). Finally, label the acid, base, conjugate acid, and conjugate base.arrow_forward
- I’m so confused please helparrow_forwardThe four bonds of carbon tetrachloride (CCl4) are polar, butthe molecule is nonpolar because the bond polarity is canceledby the symmetric tetrahedral shape. When other atoms substitutefor some of the Cl atoms, the symmetry is broken and the mol-ecule becomes polar. Use Figure to rank the fol-lowing molecules from the least polar to the most polar: CH₂Br₂,CF₂Cl₂, CH₂F₂, CH₂Cl₂, CBr₄, CF₂Brarrow_forwardWhat is the nam of this moleculearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY