
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A heating curve for the compound "benzene" is shown.
Which regions of the heating curve are where benzene is boiling?
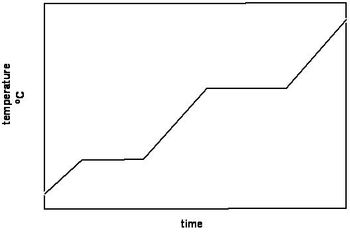
Transcribed Image Text:The image displays a graph with temperature on the vertical axis (measured in degrees Celsius, °C) and time on the horizontal axis. The graph shows a stepwise increase in temperature over time.
### Graph Explanation:
1. **Axes**
- **Y-axis (Vertical):** Represents temperature in degrees Celsius (°C).
- **X-axis (Horizontal):** Represents time.
2. **Graph Description:**
- The line graph indicates a series of linear increases in temperature over time, represented by three distinct steps.
- Each segment of the graph line is flat followed by a sharp increase, suggesting periods where the temperature remains constant, then increases suddenly.
- The graph can be divided into three main sections of increasing temperature, separated by brief periods of stability.
This type of graph might represent a process where temperature is gradually increased in stages, such as during heating experiments or industrial processes.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2 You may want to reference Section 3.7 (Pages 80-87) while completing this problem. Using the values for the heat of fusion, specific heat of water, and/or heat of vaporization, calculate the amount of heat energy in each of the following. Keep in mind that the sign of the heat involved in the state change is ignored. W Alt Jill # 3 F3 E x X C $ F4 4 R 8 % V SD F G 5 2 F5 5 HH T Fó Q Search 6 Y H 6 F7 ▼ & L Part A 7 Calculate the joules released when 145 g of steam condenses at 100 °C and the liquid cools to 35.0 °C. Express your answer to three significant figures and include the appropriate units. ASUS Value FB U HA Submit Previous Answers Request Answer * 56 X Incorrect; Try Again; 4 attempts remaining 8 BN M FO I J K D I 9 v → J 1 FIO Alt O D L BINET O ON/OFF Ctrl 0 ? P 10 : F12 I { Prt Sc [ A + = I Review | Constants | Periodic Table Insert } ] 165 Delete Backspace 1 Enter 12:06 AM 2/2/2023 ☺. ▷ Home Shift t PgUp O PgDn End Fnarrow_forwardThe heat of vaporization of water is 40.66 kJ/mol. How much heat is absorbed when 3.17 g3.17 g of water boils at atmospheric pressure?arrow_forwardCalculate the heat required to convert 22.2 g of propyl alcohol, C3H3O, from a solid at -141°C into the gaseous state at 115°C. The normal melting and boiling points of this substance are -127°C and 97°C, respectively. The heat of fusion is 86.2 J/g, and the heat of vaporization is 694 J/g. The specific heats of the solid, liquid and gaseous states are, respectively, 2.36, 2.83 and 1.76 J/g/K.arrow_forward
- The heat of vaporization of water is 40.66 kJ/mol. How much heat is absorbed when 1.59 g of water boils at atmospheric pressure?.arrow_forwardA 0.554 g sample of steam at 106.0 °C is condensed into a container with 5.20 g of water at 16.4 °C. What is the final temperature of the water mixture if no heat is lost? The specific heat of water is 4.18, the specific heat of steam is 2.01 g., and AH vap = 40.7 kJ/mol. T₁ = °Carrow_forward13) Ethanol melts at -114 C and boils at 78 °C. The enthalpy of fusion of ethanol is 5.02 kJ/mol, and its enthalpy of evaporation is 38.56 kJ/mol. The specific heats of solid and liquid ethanol are 0.97 and 2.3 J/gK, respectively. How much heat is required to convert 42.0 g of ethanol at 35 °C to the vapor phase at 78 °C?arrow_forward
- Chlorine trifluoride (ClF3) melts at -76.3 °C and boils at 11.8 °C at a constant pressure of 1 atm. What state of matter must a sample of chlorine trifluoride be in at 20°C and 1 atm?arrow_forwardCalculate the amount of heat needed to raise the temperature of 55.0 g of liquid water from 25°C to 99°C. The specific heat of liquid water is 1.00 cal/g °Carrow_forwardHeat does not have to be present in all phase changes. True O Falsearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY