
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
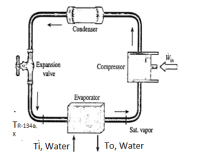
A heat pump with refrigerant-134a (R-134a) as the working fluid is used to keep a space at 21 °C by absorbing heat from geothermal water that enters the evaporator at Ti,water = 60 °C at a rate of 0.066 kg/s and leaves at To,water = 40 °C. The specific heat of liquid water is 4.18 kJ/(kg∙K). Refrigerant enters the evaporator at TR-134a = 10 °C with a quality of x = 12 % and leaves at the same pressure as saturated R-134a vapor at the same temperature. The compressor consumes 1.8 kW of power.
Determine mass flow rate, rate of heat , COP of heat pump, and Ideal minimum power input.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 7 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Q2/ Air at atmospheric pressure and 27 °C is blown across a long 4.0 cm diameter tube at a velocity of 20 m/ sec. What dimension (i.e. side) of a square duct would be needed to give the same heat transfer to the duct? Wall temperature is 50 °C in both cases. Nu=0.0266 Re0.805 Pr.1/3 Nu=0.102 Re0.675 Pr.1/3 μ/p= 17.74 x 10-6 m²/sec k=0.02711 W/m °C Pr= 0.70 For tube For square duct Re, PVD мarrow_forward4. The right figure shows a simple combustion calorimeter. The sample is ignited electrically. After a few minutes the temperature of the water and calorimeter is constant at AT higher than the starting temperature. Determine the heat of combustion of a sample from the following data: Sample mass Calorimeter mass Water mass The heat of combustion Au combustion 4 g 500 g 5000 g Sample Cvealorimeter Cvwater AT - Stirrer Oxygen O Water is defined as Ufinal products of combustion initial fuel+oxygen msample Thermometer Heavy steel bomb 0.12 cal/g °C 1.0 cal/g °C 5 °Carrow_forward6. A fermentation medium is cooled in a double pipe heat exchanger from 371.9 K to 349.7 K. The medium flows inside the inner tube and the cooling water at 288.6 K enters the annular space and flows outside the tube at 319.1 K. Calculate the heat transfer area if the heat transfer rate is 5.14 kJ/s and the overall heat transfer coefficient is 340 W/m ²K. b. For co-current flow of streams < +10arrow_forward
- A 3 meter tall aluminum tank with a 7 meter diameter is completely filled with ethanol. The empty weight of the tank is 250 kg. If the ethanol starts at 25 deg C, and there is 3000 kJ of heat available for transfer, what final temperature can be reached? And, what percentage of the heat transferred does the ethanol and aluminum each account for? (ethanol density = .789 kg/L)arrow_forwardplz write neatly, do not copy and paste solutionarrow_forwardQuestion is from the class of Heat Transfer please show all steps, Thank you.arrow_forward
- A stream of ammonia is cooled from 100oC to 20oC at a rate of 180 kg/hr in the tube side of a double-pipe counter-flow heat exchanger. Water enters the heat exchanger at 10oC at a rate of 250 kg/hr. The outside diameter of the inner tube is 3 cm and the length of the pipe is 7m. Using the log-mean temperature difference, calculate the overall heat transfer coefficient (U) for the heat exchanger. Determine the log-mean temperature difference. Determine the heat transfer coefficient for the heat exchanger. Cp for ammonia is 5234J/kgK and cp for water is 4180J/kgK.arrow_forwardA food product with 73% moisture content in a 10 cm diameter can wants to be frozen. The density of the product is 970 kg / m³, the thermal conductivity is 1.2 W / (m K), and the initial freezing temperature is -2.5 ° C. After 14 hours in the freezing medium -40 ° C, the product temperature becomes -10 ° C. Estimate the convection heat transfer coefficient of the freezing medium. Assume the can as an infinite cylinder. h = Answer W / (m² K).arrow_forwardHot brine from a geothermal well passes through 120 3 cm-O.D., 1 mm-wall- thickness, 16 m-long titanium alloy (k = 14 W/m K) tubes. Refrigerant-113 boils on the exterior of the tubes at 150°C, with an outside heat transfer coefficient of 20,000 W/m² K. The hot brine enters the tubes at 210°C, and the bulk velocity in each tube is 2 m/s. Calculate the following quantities. (i) Rep, the Reynolds number inside a tube (ii) hei, the inside heat transfer coefficient (iii) U, the overall heat transfer coefficientarrow_forward
- 10.33 ft³/min of a liquid with density (SG=1.84) is pumped 45 feet uphill. At the inlet, the pipe inner diameter is 3 in and the liquid pressure is 18 psia. At the outlet, the pipe inner diameter is 2 in and the liquid pressure is 40 psia. The friction loss in the pipe is 11.0 ft lbf/lbm- Determine the work required (hp) to pump the liquid. (Hint: gc will be helpful in this problem.)arrow_forward1. Liquid oxygen is stored in a spherical tank with D = 5 ft. The surface of the tank was isolated with insulation material A with a thickness of 8 in and outside with material B with a thickness of 0.5 ft (kA = 0.022 Btu / j.ft.oF and kB = 0.04 Btu / j.ft.oF). Tank surface temperature (–4) oC and insulation surface temperature 50oC Calculate heat transfer from air to liquid oxygen tank! 2. A 2.0 inch Schedule 40 pipe has k = 27 Btu / h.ft.oF. The fluid in the pipe has h = 30 Btu / h.ft2.oF. The outer surface of the pipe is coated with a fiber glass insulation thickness of 4 mm with k = 0.023 Btu / h.ft.oF. The convection coefficient on the outer surface of the insulation is 2.0 Btu / h.ft2.oF. The temperature of the fluid contained in the pipe is 320oF and the ambient temperature is 70oF. Calculate the heat loss per unit length of pipe! 3. Two parallel plates with a diameter of 60 cm, separated at a distance of 15 cm. The temperature on the top surface is 4 oC and the temperature on…arrow_forwardA stream of ammonia is cooled from 100oC to 20oC at a rate of 180 kg/hr in the tube side of a double-pipe counter-flow heat exchanger. Water enters the heat exchanger at 10oC at a rate of 250 kg/hr. The outside diameter of the inner tube is 3 cm and the length of the pipe is 7m. Using the log-mean temperature difference, calculate the overall heat transfer coefficient (U) for the heat exchanger. Determine the heat transfer rate between the two fluids. Determine the outlet temperature of the water. Determine the heat transfer surface area. Determine the log-mean temperature difference. Determine the heat transfer coefficient for the heat exchanger. Cp for ammonia is 5234J/kgK and cp for water is 4180J/kgK.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The