Question
Determine Ws, Q, and ΔEth for process 1→2, 2→3 and 3→1
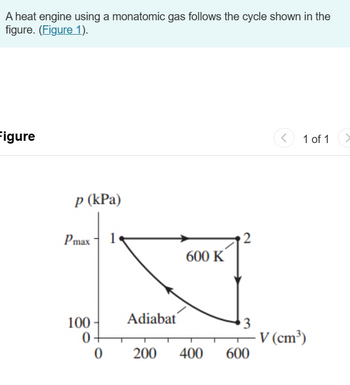
Transcribed Image Text:A heat engine using a monatomic gas follows the cycle shown in the
figure. (Figure 1).
Figure
p (kPa)
Pmax
1
2
600 K
<
1 of 1
100
Adiabat
3
0+
V (cm³)
0
200 400
600
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 5 images

Knowledge Booster
Similar questions
- Ag(s) + Pb 504(s) → Pb(s) + Agt (ac) + say (ac) Datos A H°298: 53, 22 kcal A Gers: 53,41 cal Acp298: -8,7 Cal K(T) = ? In KT = In K298 + + 1 T 55,8 8,7×103³ dł. √248 TF2 TF - 4,4 In T = +28,8-2,81 × 10-4 Tarrow_forwardThe vapor pressures of the components, A and B, in a binary solution have been modeled and found to obey хара exp(0.75 XB) A A exp(0.75x) where XÃ and are the mole fractions, and PA* and PB* are the vapor pressures of each pure substance at room temperature. (a) If PA* = 0.084 bar and the total pressure of a mixture with XA = 0.40 is 0.125 bars, what is PB*, the vapor pressure of pure B (in bars)? P = X P А P₁ = X_P B QUESTION 14 B B * * Continuation of the previous problem (b) Assuming that the vapor is an ideal gas, what is the mole fraction of component B in the vapor phase?arrow_forwardDetermine the signs of (d) Q, (e) W, and (f) ΔU associated with process BC. D) q, posative, negative, or zero E) w, posative, negative, or zero F) Δu, posative, negative, or zeroarrow_forward
- Determine how many times per second each molecule moving with rms speed would move back and forth across a 6.4 mm -long room on the average, assuming it made very few collisions with other molecules.To find vx use the equation v2=v2x+v2y+v2z and the fact that molecules have no preferred direction. vrms= 473 m/sarrow_forwardA process that takes place under constant temperature and pressure is spontaneous if AG > 0. True Falsearrow_forwardCurrent Attempt in Progress Compute (a) the number of moles and (b) the number of molecules in 2.2 cm of an ideal gas at a pressure of 88 Pa and a temperature 3 of 220 K. (a) Number Units (b) Number i Units >arrow_forward
arrow_back_ios
arrow_forward_ios