
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
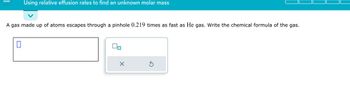
Transcribed Image Text:Using relative effusion rates to find an unknown molar mass
A gas made up of atoms escapes through a pinhole 0.219 times as fast as He gas. Write the chemical formula of the gas.
0
X
Ś
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Sulfur hexafluoride gas is collected at -2.0 °C in an evacuated flask with a measured volume of 5.0 L. When all the gas has been collected, the pressure in the flask is measured to be 0.070 atm . Calculate the mass and number of moles of sulfur hexafluoride gas that were collected. Be sure your answer has the correct number of significant digits. mass: g mole: molarrow_forwardBoron trifluoride gas is collected at 1.0 °C in an evacuated flask with a measured volume of 15.0 L. When all the gas has been collected, the pressure in the flask is measured to be 0.420 atm . Calculate the mass and number of moles of boron trifluoride gas that were collected. Be sure your answer has the correct number of significant digits. mass: mole: molarrow_forwardThe balanced equation for the chemical equation is Mg+2HCl->MgCl2+H2 If the student used an excess of hcl to dissolve 0.045 grams of pure Mg ribbon, and collected the gas in a test tube over water how much H2 gas could she produce, calculate the answer in moles.arrow_forward
- A sample of hydrogen gas at a pressure of 0.802 atm and a temperature of 29.8 c, occupies a volume of 18.2 L. If the gas is allowed to expand at constant temperature to a volume of 27.5 L, the pressure of the gas sample will be atm.arrow_forwardThe pressure of a gas results from which of the following? gas particles colliding with the container walls. the sum of the volumes of the particles being larger than the container volume. gas particles colliding with each other. The temperature of a gas is a measure of which of the following? the amount of space between gas particles. the kinetic energy of the gas particles. the number of gas particles. the volumes of the gas particles.arrow_forwardA 4.72 mol sample of freon gas was placed in a balloon. Adding 3.50 mol of freon gas to the balloon increased its volume to 22.5 L. What was the initial volume of the balloon?arrow_forward
- If 9.00 g helium gas is added to a 1.00 L balloon containing 1.00 g of helium gas, what is the new volume of the balloon? Assume no change in temperature or pressure. Enter your answer in the box provided. L.arrow_forwardConsider a gas sample with an initial pressure of 533 torr and a volume of 10.5 L. What is the pressure of the gas when the volume of the gas increases by a factor of 2.21, as the temperature of the gas remains constant.arrow_forwardWhich of the following properties is unique to gases compared to other states of matter ?arrow_forward
- A 1.23 mol sample of xenon gas is collected at a pressure of 521 mmHg and a temperature of 16.0°C. The volume of the sample is L.arrow_forward28 29 30 31 32 33 34 35 36 37 38 39 Sulfur tetrafluoride gas is collected at -6.0 °C in an evacuated flask with a measured volume of 40.0 L. When all the gas has been collected, the pressure in the flask is measured to be 0.180 atm . Calculate the mass and number of moles of sulfur tetrafluoride gas that were collected. Round your answer to 3 significant digits. mass: g x10 mole: mol Submit Assignment Continue O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center | Accessibility ShoL IMG-6096.jpg IMG-6095.jpg IMG-6097.jpg IMG-6098.jpg IMG-6099.jpg MacBook Air DII F11 80 F9 F10 F7 F8 F6 F5 esc F4 F3 F1 F2 & @ # $ % 4 5 6 7 8 2 3arrow_forwardA gas made up of atoms escapes through a pinhole 1.26 times as fast as O2 gas. Write the chemical formula of the gas.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY