
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
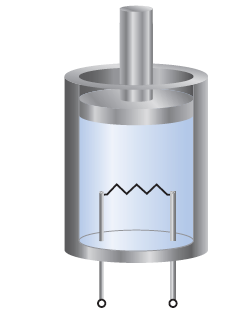
A gas is confined to a cylinder fitted with a piston and an
electrical heater, as shown here:
Suppose that current is supplied to the heater so that 100 J of
energy is added. Consider two different situations. In case (1)
the piston is allowed to move as the energy is added. In case
(2) the piston is fixed so that it cannot move. (a) In which
case does the gas have the higher temperature after addition
of the electrical energy? (b) Identify the sign (positive, negative,
or zero) of q and w in each case? (c) In which case is ΔE
for the system (the gas in the cylinder) larger?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each of the following processes, indicate whether the signs of S and H are expected to be POSITIVE, NEGATIVE or ABOUT ZERO.(a) A mixture of liquid oxygen and liquid hydrogen is turned into water vapor.For this process S should be______ and H should be ______ .(b) Two moles of a cold gas react to form two moles of a different, hot gas.For this process S should be_____ and H should be _____ .(c) Solid sulfur burns in pure oxygen generating sulfur dioxide gas.For this process S should be_______ and H should be ______ .arrow_forwardAt 25°C, 6.816 koal is released in the detonation of 0.001 mole of solid TNT, in a constant- velume calorimeter. Given that in this reaction 1 mole of TNT yields 5 moles of gaseous Na and CO, together with solid and liquid products of negligible volume, calculate A and AA per mole of TNT exploded.arrow_forwardThe figure shows a PV diagram for an engine that uses a monatomic ideal gas as the working substance. The temperature at point A is 470.0 K.(a) How much net work does this engine do per cycle?(b) Assuming that the efficiency of the engine is 0.444, what is the heat input into the gas per cycle?(c) How much heat is exhausted per cycle?(d) How many moles of gas are used in this engine?(e) What is the total change in internal energy of this gas in one cycle?arrow_forward
- 18.25arrow_forwardPlease don't provide handwritten solution ....arrow_forward2.25 moles of an ideal gas expands irreversibly from a pressure of 1.24 bars to a pressure of 0.239 bars, with its initial and final temperatures both at T = 300 K. No work is done in this process (you can imagine that it is a free expansion into an evacuated space, if you wish). (a) Compute the change in volume for the gas, AV (in L). 189.54 QUESTION 16 Continuation of the previous problem (b) Compute AG (in kJ) for this expansion. (You should compute AS as part of this evaluation.)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY