
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
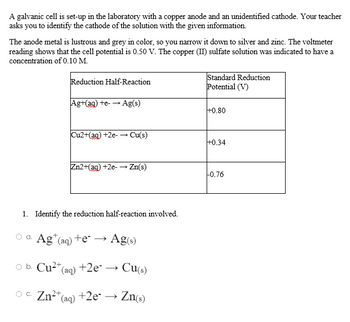
Transcribed Image Text:A galvanic cell is set-up in the laboratory with a copper anode and an unidentified cathode. Your teacher
asks you to identify the cathode of the solution with the given information.
The anode metal is lustrous and grey in color, so you narrow it down to silver and zinc. The voltmeter
reading shows that the cell potential is 0.50 V. The copper (II) sulfate solution was indicated to have a
concentration of 0.10 M.
Reduction Half-Reaction
Standard Reduction
Potential (V)
Ag+(ag) +e- → Ag(s)
+0.80
Cu2+(ag) +2e-→ Cu(s)
+0.34
Zn2+(aq) +2e-→ Zn(s)
-0.76
1. Identify the reduction half-reaction involved.
a.
Ag (aq) +e → Ag(s)
O
b. Cu²+ (aq) +2e*. →
Cu(s)
OC. Zn²+ (aq) +2e- → Zn(s)
c.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A certain half-reaction has a standard reduction potential -0 red = = +0.90 V. An engineer proposes using this half-reaction at the cathode of a galvanic cell that must provide at least 0.70 V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the cathode of the cell. ロ→ロ 0 Is there a minimum standard reduction potential that the half-reaction used at the anode of this cell can have? yes, there is a minimum. ☐v red If so, check the "yes" box and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, check the "no" box. no minimum Ś ? 0 Is there a maximum standard reduction potential that the half-reaction used at the anode of this cell can have? yes, there is a maximum. Ov red If so, check the "yes" box and calculate the maximum. Round your answer to 2 decimal places. If there is no upper limit, check the "no" box. no maximum E E = = Xarrow_forward. At 25 C, the standard hydrogen electrode (SHE) has a half-cell potential that is defined to be 0.00 volts. If the pH is increased at this electrode (keeping everything else the same), at what pH will the half-cell potential be equal to -0.521 volts?arrow_forwardThe following spontaneous reaction occurs when metallic zinc is dipped into a solution of copper sulfate. Zn (s) +Cu^ 2+ (aq) ----> Zn^ 2+ (aq) +Cu (s) Describe a galvanic cell that could take advantage of this reaction. What are the half-cell reactions? Make a sketch of the cell and label the cathode and anode, the charges on each electrode , the direction of ion flow, and the direction of electron flow .arrow_forward
- A galvanic (voltaic) cell consists of an electrode composed of aluminum in a 1.0 M aluminum ion solution and another electrode composed of gold in a 1.0 M gold(III) ion solution, connected by a salt bridge. Calculate the standard potential for this cell at 25 °C. Refer to the list of standard reduction potentials. Excell Varrow_forwardA certain metal M forms a soluble nitrate salt MNO33 . Suppose the left half cell of a galvanic cell apparatus is filled with a 5.00M solution of MNO33 and the right half cell with a 25.0mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 35.0°C. What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode.Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.arrow_forwardThe silver zinc battery is used in hearing aids. Explain the fundamental redox reactions that occur in this battery and calculate the theoretical cell potential for this system.arrow_forward
- 3.5. Diagrams A and B show two different sample injection systems for electrophoresis separation. Describe their mode of operation. Assuming that our sample in both diagrams comprises the composition of anions, cations, and neutral molecules, identify them in order of their movement in the capillary. Sample ►Vacuum Buffer Buffer Figure 4: Injection systems. B A (312)arrow_forwardA galvanic cell was constructed with a nickel electrode that was dipped into 1.1 M NiSO4 solution and a chromium electrode that was immersed into a solution containing Cr3+ at an unknown concentration. The potential of the cell was measured to be 0.557 V, with the chromium serving as the anode. The standard cell potential for this system was determined to be 0.487 V. What was the concentration of Cr3+ in the solution of unknown concentration?arrow_forwardO ELECTROCHEMISTRY = Understanding concentration cells ODDOD 3/5 A certain metal M forms a soluble nitrate salt MNO3. Suppose the left half cell of a galvanic cell apparatus is filled with a 1.50 M solution of MNO3 and the right half cell with a 150. mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 30.0 °C. Which electrode will be positive? Oleft right ? What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. 0 Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY