
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
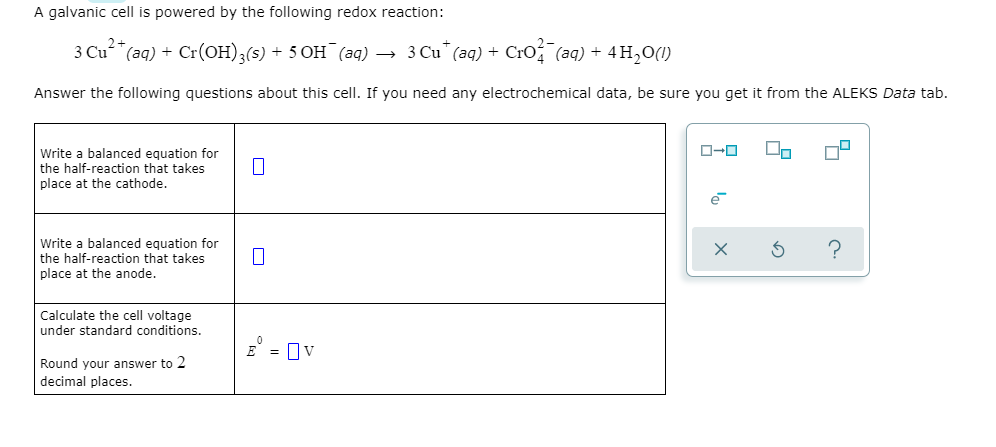
Transcribed Image Text:A galvanic cell is powered by the following redox reaction:
3 Cu (aq) + Cr(OH);(s) + 5 OH (aq) → 3 Cu" (aq) + Cro (aq) + 4H,0(1)
Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab.
Write a balanced equation for
the half-reaction that takes
place at the cathode.
Write a balanced equation for
the half-reaction that takes
place at the anode.
Calculate the cell voltage
under standard conditions.
E = Ov
Round your answer to 2
decimal places.
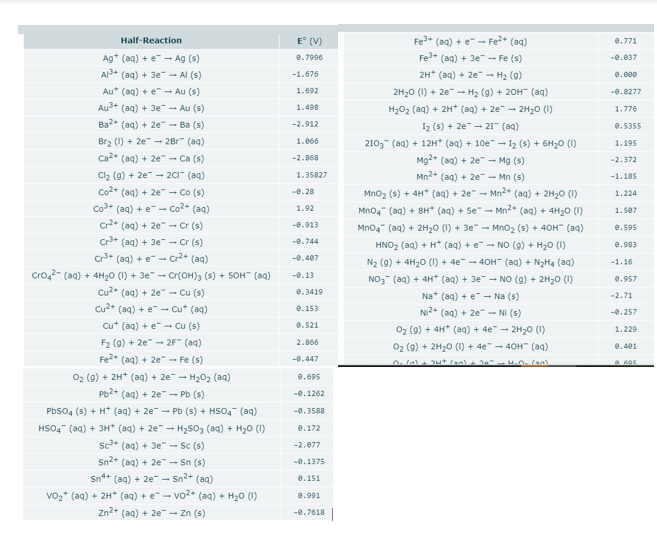
Transcribed Image Text:Half-Reaction
E (V)
Fe* (aq) + e" - Fe2* (aq)
0.771
Ag* (aq) + e - Ag (s)
Fe+ (aq) + 3e" - Fe (s)
e.7996
-e.037
Al3+ (aq) + 3e" - Al (s)
2H* (aq) + 2e - H2 (9)
2H20 (1) + 2e - H2 (g) + 20H" (aq)
-1.676
e.eee
Au* (aq) + e" - Au (s)
1.692
-e.8277
Au+ (aq) + 3e" - Au (s)
1.498
H202 (aq) + 2H* (aq) + 2e - 2H20 (1)
1.776
Ba2* (aq) + 2e" - Ba (s)
-2.912
I2 (s) + 2e" - 21" (aq)
e.5355
Brz (1) + 2e" - 2Br" (aq)
1.066
2103 (aq) + 12H* (aq) + 10e - Iz (s) + 6H20 (1)
Mg2+ (aq) + 2e" - Mg (s)
Mn2+ (aq) + 2e" - Mn (s)
1.195
Ca2* (aq) + 2e" - Ca (s)
-2.868
-2.372
Clz (9) + 2e - 20 (aq)
1.35827
-1.185
Co2* (aq) + 2e" - Co (s)
-e.28
Mno2 (s) + 4H* (aq) + 2e - Mn2* (aq) + 2H20 (1)
Mn04" (aq) + 8H* (aq) + 5e- Mn2+ (aq) + 4H20 (1)
1.224
Co+ (aq) + e- Co2* (aq)
1.92
1.507
Cr2* (aq) + 2e" - Cr (s)
-e.913
Mno4 (aq) + 2H20 (1) + 3e- Mno2 (s) + 40H" (aq)
HNO2 (aq) + H* (aq) + e- NO (g) + H20 (1)
N2 (g) + 4H20 (1) + 4e - 40H- (aq) + N2H4 (aq)
e.595
Cr+ (aq) + 3e" - Cr (s)
-e.744
e.983
Cr* (aq) + e- Cr2* (aq)
Cro,2- (ag) + 4H20 (1) + 3e" - Cr(OH)3 (s) + SOH- (aq)
-0.407
-1.16
-e.13
NO3 (aq) + 4H* (aq) + 3e - NO (9) + 2H20 (1)
e.957
Cu2* (aq) + 2e" - Cu (s)
Cu2+ (aq) + e - Cu* (aq)
e.3419
Na* (aq) + e - Na (s)
-2.71
e.153
NI2+ (aq) + 2e" - NI (s)
-0.257
Cu* (aq) + e" - Cu (s)
e.521
02 (9) + 4H* (aq) + 4e" - 2H2O (1)
1.229
F2 (9) + 2e" - 2F" (aq)
2.866
02 (9) + 2H20 (1) + 4e" - 40H" (aq)
e.401
Fe* (aq) + 2e - Fe (s)
-0.447
O2 (9) + 2H* (aq) + 2e - H202 (aq)
e.695
Pb2* (aq) + 2e - Pb (s)
-e.1262
Pbso4 (s) + H* (aq) + 2e - Pb (s) + HSO4 (aq)
-0.3588
HSO4" (aq) + 3H" (aq) + 2e - H2S03 (aq) + H20 (1)
sc* (aq) + 3e" - Sc (s)
Sn2* (aq) + 2e" - Sn (s)
0.172
-2.077
-e.1375
Sn+ (aq) + 2e" - Sn2+ (aq)
e.151
vo2* (aq) + 2H* (aq) + e- vo2+ (aq) + H20 (1)
e.991
Zn2+ (aq) + 2e" - Zn (s)
-0.7618|
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Please explain and give the correct answers.arrow_forwardCould you please solve the last two questions from the second picture?arrow_forwardConsider the galvanic cell based on the following half-reactions. Ag+ + e− → Ag ℰ° = +0.80 V Fe2+ + 2 e− → Fe ℰ° = −0.44 V (a) Determine the overall cell reaction and calculate ℰ ° cell . (b) Calculate ΔG° and K for the cell reaction at 25°C. (c) Calculate ℰcell at 25°C when [Ag+ ] = 1.0 ✕ 10−4 M and [Fe2+ ] = 1.0 ✕ 10−2 M.arrow_forward
- A galvanic cell is powered by the following redox reaction: 2+ + 3 Cu²+ (aq) + Cr(OH)3(s) + 5OH¯(aq) → 3 Cut (aq) + Cro (aq) + 4H₂O(1) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. Round your answer to 2 decimal places. 0 0 0 E = v V ロ→ロ X 5arrow_forwardA galvanic cell is powered by the following redox reaction: 3 Br₂(1) + 2NO(g) + 4H₂O(1) - 6 Br (aq) + 2NO3(aq) + 8H (aq) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. Round your answer to 2 decimal places. 0 0 E = v 00 Śarrow_forwardDesigning a galvanic cell from tw... A chemist designs a galvanic cell that uses these two half-reactions: standard reduction |MnO4(aq)+2H₂O(l)+3e¯ → MnO₂(S)+4OH(aq) Cl₂(g) +2e 2 Cl(aq) Answer the following questions about this cell. Write a balanced equation for the half-reaction that 0 happens at the cathode. Write a balanced equation for the half-reaction that happens at the anode. Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written. Do you have enough information to calculate the cell voltage under standard conditions? If you said it was possible to calculate the cell voltage, do so and enter your answer here. Round your answer to 2 significant digits. 0 half-reaction O Yes O No ☐v - potential Ered = +0.59 V = +1.359 V ロ→ロ X x10 Sarrow_forward
- Consider a galvanic electrochemical cell constructed using Cr/Cr³+ and Zn/Zn²+ at 25 °C. The following half-reactions are provided for each metal: Cr³+(aq) + 3 e →→ Cr(s) E°red = -0.744 V Zn²+ (aq) + 2 e →→ Zn(s) Eºred = -0.763 V Write the balanced equation for the overall reaction in acidic solution. 3 Zn(s) + 2 Cr³+ (aq) → 3 Zn²+(aq) + 2 Cr (s) 1 1 + 4- Reset 2 H₂O+ 0 3_ ( ) 3 03 04 4 H H+ 5 5 11 Zn 6 6 ²+ 7 07 (s) H₂O ● 3+ (1) 8 9 0 e 4+ x H₂O 9 (g) (aq) □o Cr Deletearrow_forwardA galvanic cell is powered by the following redox reaction: 2+ 3+ O,(g) + 4H'(aq) + 4 Fe´'(aq) 2 H20(1) + 4 Fe"(aq) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. E° = [v Round your answer to 2 decimal places.arrow_forwardA galvanic cell is powered by the following redox reaction: 2 Br,(1) + N,H,(aq) + 4OH (aq) 4 Br (aq) + N,(g) + 4 H,O(1) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. Write a balanced equation for the half-reaction that takes place at the cathode. ローロ e Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. E - Round your answer to 2 decimal places. のarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY