
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
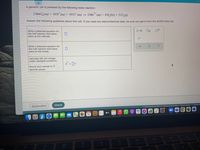
Transcribed Image Text:A galvanic cell is powered by the following redox reaction:
2 MnO (aq) + 16H"(aq) + 10 Cl (aq) → 2Mn (aq) + 8 H,0(1) + 5 Cl,(g)
2+
Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab.
Write a balanced equation for
the half-reaction that takes
place at the cathode.
e
Write a balanced equation for
the half-reaction that takes
place at the anode.
Calculate the cell voltage
under standard conditions.
E = v
Round your answer to 2
decimal places.
Check
Explanation
2022 McGraw Hill LLC. All Riahts Reserved. Terms of Use Privacv C
MAR
étv
16
80
F3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- + 2+ MnO 4(aq) +8 H (aq) +5e → Mn(aq) + 4H₂O(1) Write a balanced equation for the half-reaction that happens at the cathode. Write a balanced equation for the half-reaction that happens at the anode. Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written. Answer the following questions about this cell. Do you have enough information to calculate the cell voltage under standard conditions? 3+ If you said it was possible to calculate the cell voltage, do so and enter your answer here. Round your answer to significant digits. Fe half-reaction 0 (aq) + e Yes No V 2+ Fe (aq) standard reduction potential red 'red = +1.51 V = +0.771 V ローロ x 90 x10 Sarrow_forwardPlease explain and give the correct answers.arrow_forwardA galvanic cell is powered by the following redox reaction: 2+ 2+ MnO₂ (s) + 4H* (aq) + Zn(s) Mn²+ (aq) + 2 H₂O(1) + Zn²+ (aq) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. Round your answer to 2 decimal places. 0 £ - Ov E V ローロ X Garrow_forward
- Could you please solve the last two questions from the second picture?arrow_forwardA galvanic cell is powered by the following redox reaction: 2+ 2 Cro(aq) + 8 H₂O(1) + 3 Zn(s) 2 Cr(OH)3(s) + 10 OH¯(aq) + 3 Zn¹¹(aq) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. Round your answer to 2 decimal places. 0 □ E = v ロ→ロ e X Śarrow_forwardA galvanic cell is powered by the following redox reaction: 3 Br₂(1) + 2NO(g) + 4H₂O(1) - 6 Br (aq) + 2NO3(aq) + 8H (aq) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. Round your answer to 2 decimal places. 0 0 E = v 00 Śarrow_forward
- An electrochemical cell is made of two electrodes: (Zn2+/Zn) and (Ag+/Ag). Use the standard reduction potentials to answer the following questions. What is the standard potential of the cell and which electrode is the cathode and which one is the anode? Write a net redox equation for this electrochemical cell.arrow_forwardSuppose the galvanic cell sketched below is powered by the following reaction: 3 Mn(s)+2 Fe(NO3),(aq) 3 Mn(NO3),(aq)+2 Fe(s) E1 E2 S1 S2 Write a balanced equation for the half-reaction that happens at the cathode of this cell. Write a balanced equation for the half-reaction that happens at the anode of this cell. e Of what substance is E1 made? Of what substance is E2 made? What are the chemical species in solution S1? What are the chemical species in solution S2? O OO OO Oarrow_forwardCro (aq) + 4 H₂O(1)+3e Write a balanced equation for the half-reaction that happens at the cathode. Write a balanced equation for the half-reaction that happens at the anode. Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is swer the following questions about this cell. spontaneous as written. Do you have enough information to calculate the cell voltage under standard conditions? 2+ Cu (aq) + e If you said it was possible to calculate the cell voltage, do so and enter your answer 0 half-reaction 0 2 Cu²+ (aq) + e → Cu (aq) Yes No + 0.28 V Cu (aq) Cr(OH)3(s)+5 OH (aq) standard reduction potential E .0 'red Eº ´red = +0.153 V -0.13 V ロ→ロ e × x10 Śarrow_forward
- A galvanic cell is powered by the following redox reaction: 2+ 3+ O,(g) + 4H'(aq) + 4 Fe´'(aq) 2 H20(1) + 4 Fe"(aq) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. E° = [v Round your answer to 2 decimal places.arrow_forwardA galvanic cell is powered by the following redox reaction: 2 Br,(1) + N,H,(aq) + 4OH (aq) 4 Br (aq) + N,(g) + 4 H,O(1) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. Write a balanced equation for the half-reaction that takes place at the cathode. ローロ e Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. E - Round your answer to 2 decimal places. のarrow_forwardA chemist designs a galvanic cell that uses these two half-reactions: 3+ 2+ Fe (aq)+e → Fe (aq) Cro (aq) + 4H₂O(1)+3e¯ Cr(OH)3(s)+5 OH(aq) Answer the following questions about this cell. Write a balanced equation for the half-reaction that happens at the cathode. Write a balanced equation for the half-reaction that happens at the anode. Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is half-reaction spontaneous as written. 0 standard reduction potential Ed=+0.771 V 'red E 'red = -0.13 V ロ→ロ × x10 5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY