
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
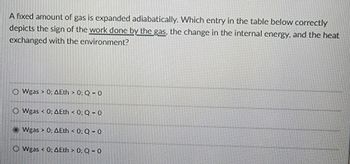
Transcribed Image Text:A fixed amount of gas is expanded adiabatically. Which entry in the table below correctly
depicts the sign of the work done by the gas, the change in the internal energy, and the heat
exchanged with the environment?
O Wgas > 0: AEth > 0: Q-0
ⒸWgas < 0: AEth < 0: Q-0
Wgas > 0: AEth < 0: Q-0
Wgas 0: AEth > 0: Q-0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The volume of an ideal gas is increased from 1 m3 to 3 m3 at a constant pressure of 1000 Pa How much work is done by the gas in the expansion? W= _________ J If no heat has been added or removed, what is the change in internal energy of the gas? DU = ________ Jarrow_forwardThe picture shows a pV diagram for an ideal gas in which its pressure tripled from a to b when 804 J of heat was put into the gas. Work done on or by the gas between a and b= 0 W Delta U=804 J a) What is the temperature of the gas at point bb in terms of its temperature at a, Ta?arrow_forwardA car tire contains 0.0390 m3 of air at a pressure of 2.20×105 N/m2 (about 32 psi). How much more internal energy does this gas have than the same volume has at zero gauge pressure (which is equivalent to normal atmospheric pressure)?arrow_forward
- A gas expands from I to F in the figure below. The energy added to the gas by heat is 302 J when the gas goes from I to Falong the diagonal path. Three paths are plotted on a PV diagram, which has a horizontal axis labeled V(liters), and a vertical axis labeled P (atm). The green path starts at point I (2,4), extends vertically down to point B(2,1), then extends horizontally to point F (4,1). The blue path starts at point I (2,4), and extends down and to the right to end at point F (4,1). The orange path starts at point I(2,4), extends horizontally to the right to point A (4,4), then extends vertically down to end at point F(4,1). (a) What is the change in internal energy of the gas?J(b) How much energy must be added to the gas by heat for the indirect path IAF to give the same change in internal energy?Jarrow_forwardAn ideal gas undergoes an isobaric process at a pressure of 200 kPa. The volume of the gas, which contains 35 mol of particles, expands from 0.65m3 to a final volume of 1.4m3. (a) How much work is done by/on the gas during this process? (b) What are the initial and final temperatures of the gas? (c) By how much does the internal energy of the gas change? (d) How much heat was gained/lost by the gas?arrow_forwardThe heat engine shown in the figure uses 2.0 mol of a monatomic gas as the working substance. (Figure 1) igure p (kPa) 600- 400 200 0 0 0.025 0.050 V (m³) 1 of 1 Part E part. What is the engine's thermal efficiency? Express your answer using two significant figures. η = Submit VE ΑΣΦ Request Answer ? %arrow_forward
- A gas expands from I to F in the figure below. The energy added to the gas by heat is 212 J when the gas goes from I to F along the diagonal path. Three paths are plotted on a PV diagram, which has a horizontal axis labeled V (liters), and a vertical axis labeled P (atm). The green path starts at point I (2,4), extends vertically down to point B (2,1), then extends horizontally to point F (4,1). The blue path starts at point I (2,4), and extends down and to the right to end at point F (4,1). The orange path starts at point I (2,4), extends horizontally to the right to point A (4,4), then extends vertically down to end at point F (4,1). (a) What is the change in internal energy of the gas? Use the relations between various features of the graph and the work done on the gas to find the energy added by work and then use your result to find the change in internal energy of the gas. J(b) How much energy must be added to the gas by heat for the indirect path IAF to give the same change in…arrow_forwardThe PV diagram shows the compression of 40.9 moles of an ideal monoatomic gas from state A to state B. Calculate Q, the heat added to the gas in the process A to B. Data: PA= 1.90E+5 N/m2 VA= 1.83E+0 m3 PB= 1.01E+5 N/m2 VB= 8.90E-1 m3›44arrow_forwardAn ISOBARIC process is one in which the PRESSURE REMAINS CONSTANT. Answer the following questions regarding the isobaric process.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON