
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Predict the effect of increasing the container volume on theamounts of each reactant and product in the following reactions:
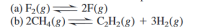
Transcribed Image Text:(a) F2(g) 2F(g)
(b) 2CH4(8) C,H2(g) + 3H2(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following reaction where Kc CO(g) + Cl₂(g) CoCl₂(g) = 77.5 at 600 K: A reaction mixture was found to contain 4.16×10-² moles of CO(g), 4.43×10-² moles of Cl₂(g) and 0.106 moles of COCl₂(g), in a 1.00 Liter container. Indicate True (T) or False (F) for each of the following: TF 1. In order to reach equilibrium COCI₂(g) must be consumed. 2. In order to reach equilibrium Kċ must decrease. 3. In order to reach equilibrium CO must be consumed. 4. Qc is greater than Kc. 5. The reaction is at equilibrium. No further reaction will occur.arrow_forwardIn the gaseous form, SO3 is a significant air pollutant, being the primary agent in acid rain. SO3 can further react with nitrogen monoxide to create NO2 which is also an air pollutant, as seen in the reaction below: SO3 (g) + NO (g) – NO2 (g) + SO2 (g) At 298K, the concentrations of the four gasses were found to be: [NO] = 0.18 mol/L [SO3] = 0.52 mol/L [SO2] = 1.34 mol/L [NO2]= 2.56 mol/L %3Darrow_forwardN2(g) + 3H2(g) + 2NH3(g) At 500°C, the value of Kc for this reaction is 0.40. The following concen- trations of gases are present in a container at 500°C: [N2(g)] = 0.10 mol/L, [H2(g)] = 0.30 mol/L, and [NH3(g)] = 0.20 mol/L. Is this mixture of gases at equilibrium? If not, in which direction will the reaction go to reach equilibrium? Is this mixture of gases at equilibrium: If "no", in which direction will the reaction go: (yes/no) (left/right/no-shift)arrow_forward
- For the reaction: 2 NO2 (g) + O2 (g) --> 2 NO3 (g) at 923 °C , Kc is 42.5. If 0.0500 moles of NO2 (g), 0.122 moles of O2 (g) and 0.300 moles of NO3 (g) are mixed in a 1.00 liter container at 923 °C, in what direction will the reaction proceed? (Show your calculation to prove that your answer is not a guess.)arrow_forwardConsider the following reaction where Kc = 55.6 at 698 K. H2(g) + I2(g) ----> 2HI(g) A reaction mixture was found to contain 4.26×10-2 moles of H2(g), 4.04×10-2 moles of I2(g) and 0.270 moles of HI(g), in a 1.00 liter container. Is the reaction at equilibrium?If not, what direction must it run in order to reach equilibrium?The reaction quotient, Qc, equals _____ The reaction (fill in the blank) ____ A. must run in the forward direction to reach equilibrium.B. must run in the reverse direction to reach equilibrium.C. is at equilibrium.arrow_forwardAs you are walking across your laboratory, you notice a 5.25 L flask containing a gaseous mixture of 0.0205 mole NO2 (9) and 0.750 mol N2O4 (q) at 25°C. 4 (g) Is this mixture at equilibrium? If not, will the reaction proceed towards forming more products, or more reactants? N2O4 4 (9) → 2NO2 (9) Ko = 4.61 x 103 at 25°Carrow_forward
- 6. Consider the following reaction: CO(g) + H2O(g) CO2(g) + H2(g) Ko = 0.58 at 1273 K* A sealed 4.00L vessel initially contains 1.00 mole CO2(g), 1.00 mole H2(g), 0.600 moles CO(g), and 0.600 moles of H2O(g). Calculate the moles of H2(g) present at equilibrium. The NG Reference: General Chemistry 8th Ed: Ebbing and Gammon: 2005 by Houghton Mifflin. New York. page 654, print 68arrow_forwardA methanol synthesis reaction will be run in a container with a moveable piston to allow for changes in volume. The gas phase reaction is illustrated below: CO(g) + 2H2(g) = CH3OH(g) At equilibrium, the reaction is run at 400K in a 10L flask; there are 3.09 moles of CO and 1.11 moles H₂ initially. At constant volume, 0.830 mol H2(g) is added to the reaction. Solve for the partial pressures of the gases in the container when the reaction returns to equilibrium. Really not sure how I should solve this problem, please help me out my showing step by step what I should do. Thanks :)arrow_forwardPlease show all steps clearly to this problem. Thanksarrow_forward
- Calculate the pressure equilibrium constant KP for the equilibrium between nitrogen monoxide, oxygen, and nitrogen dioxide has the final temperature of the mixture round your answer to two significant digits.arrow_forwardIn a certain experiment 7.50 mile of NH3 was placed in an empty 1.0 liter flask at 900C. After equilibrium was reached, 0.50 mile of N2 was found to be in the flask (along with other gases). Calculate the [NH3] and [H2] at equilibrium and the numerical value of Kc. 2NH3 (g) = N2(g) + 3H2(g)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY