
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:II
uuntunyu00003/Tuliscreen/12868783/View
McGraw-Hill Campus - ALEKS Science
O GASES
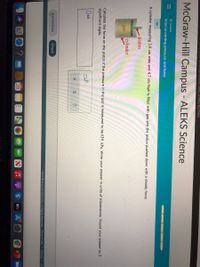
Interconverting pressure and force
A cylinder measuring 3.9 cm wide and 4.7 cm high is filled with gas and the piston pushed down with a steady force.
piston
cylinder
gas
Calculate the force on the piston if the pressure in the gas is measured to be 639. kPa. Write your answer in units of kilonewtons. Round your answer to 2
significant digits.
Explanation
Check
2021 McGraw-Hill Education. All Rights Reserved. Terms of Use
Privacy Ac
28
étv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following balanced equation. 2 Sb (s) + 3 H2O (g) → Sb2O3 (s) + 3 H2 (g) 5.0 L of H2O gas, with a pressure of 1.29 atm and a temperature of 96.7 °C reacted. How many mol of Sb2O3 were formed? Write out the problem on paper showing all conversion factors, unit cancellations, calculations, s.f., etc. Answer the questions related to the setup and calculation for this problem. Be sure to use our periodic table to calculate any molar masses needed (rounded to proper number of decimal places), otherwise your values might be slightly off and answers may be marked as incorrect. Abbreviate units as follows: grams = g, moles = mol, liters = L, atmospheres = atm What temperature (with proper s.f.) is entered into the equation? Enter the value in the first blank and the units in the second blank. Calculate the mol of H2O reacted (enter value only, with proper s.f.). mol Use the three blanks to enter the number, unit, and substance (in this order) that appears in…arrow_forwardFind the density of Freon-11 (CFC13) at 110.0 °C and 6.87 atm. Be sure your answer has the correct number of significant figures. Note: Reference the Fundamental constants table for additional information. g L 0x1.8 Xarrow_forwardOne of the steps to sweeten sour gas using the Claus process is reacting hydrogen sulfide gas with sulfur dioxide gas to produce water vapour and sulfur. 8.43 kL of hydrogen sulfide at 175 kPa and 250 °C reacts with excess sulfur dioxide. Calculate the mass, in kg, of sulfur produced.Do not show your work in the space provided.Record only your final answer with the correct number of significant digits and the proper units.arrow_forward
- How hot must you heat 369 mL of gas at 33.3°C for it to expand to 3.6 L? Express your answer in Kelvin and round to 1 decimal place. Be sure to use the proper abbreviation of the UNITSarrow_forwardA sample of helium gas was collected over water. The volume of gas was 10.54 L, room pressure was 103.9 kPa, and the temperature was 24.0°C. What was the mass of the helium gas?arrow_forwardExpress 28.5 cm Hg in units of atm. Enter your answer in the provided box. atmarrow_forward
- Use the References to access important values if needed for this question. A mixture of neon and argon gases, at a total pressure of 882 mm Hg, contains 3.46 grams of neon and 2.27 grams of argon. What is the partial pressure of each gas in the mixture? PNe= mm Hg PAr= mm Hg I| 11arrow_forwardIn degrees Celsius determine the temperature of a stored gas.arrow_forwardConvert 84.00 Fahrenheit to Kelvin.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY