
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
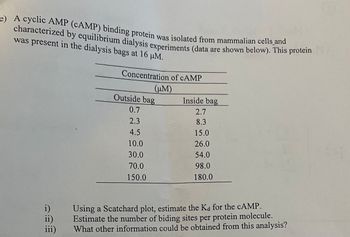
Transcribed Image Text:Here is the transcribed content from the image suitable for an educational website:
---
**Equilibrium Dialysis and cAMP Binding Protein Analysis**
A cyclic AMP (cAMP) binding protein was isolated from mammalian cells and characterized using equilibrium dialysis experiments. The data from these experiments is provided below. The protein was present in the dialysis bags at a concentration of 16 μM.
---
**Concentration of cAMP (μM)**
| Outside Bag | Inside Bag |
|-------------|------------|
| 0.7 | 2.7 |
| 2.3 | 8.3 |
| 4.5 | 15.0 |
| 10.0 | 26.0 |
| 30.0 | 54.0 |
| 70.0 | 98.0 |
| 150.0 | 180.0 |
---
**Analysis Questions:**
i) Using a Scatchard plot, estimate the Kd for the cAMP.
ii) Estimate the number of binding sites per protein molecule.
iii) What other information could be obtained from this analysis?
---
**Instructions for Further Analysis:**
To analyze the binding characteristics of the cAMP-binding protein, you would typically use a Scatchard plot to determine the dissociation constant (Kd) and the number of binding sites. Plot the ratio of bound to free ligand versus the concentration of bound ligand. This analysis will provide insights into the affinity and capacity of the protein for cAMP, valuable for understanding its functional role in cellular processes.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Similar questions
- A fictional cubed-shaped bacterium, Bacterius cubis, occupies a volume of 2.8 femtoliters. This particular type of bacteria is known to communicate with its own species by secreting a small molecule called bactoX (MW=122.4 g/mol). A. Each bacterium contains 8850 bactoX molecules that can be secreted. How many moles of bactoX are present in a 1.2 μL sample volume that contains 3.234×102 bacterial cells? moles: _______ mol B. Calculate the molarity (mol/L) of bactoX in the 1.2 μL sample volume if all of the bacteria were to simultaneously secrete all of the bactoX molecules contained within their cell bodies into the vial. concentration: ________ M C. Scientists discovered that bactoX will induce a luminescence response in B. cubis when the concentration in a sample reaches 7.00×10 -12 M. How many molecules of bactoX must be present in solution to initiate luminescence in a 1.8 μL sample? How many individual bacteria cells does this represent? The number of bactoX…arrow_forwardA flat (not spherical) tissue engineered skin (thickness 2.5 mm) is attached to the bottom of a dish and cultured with cell media atop it (which has an oxygen concentration of 0.15 mol/m3). The diffusivity of oxygen in the skin is 3.4 E-9 m2/s, and according to experiments that you’ve performed, you want to make sure that the concentration of oxygen in the device doesn’t fall below 0.06 mol/m3. (Below that concentration harms the cells.) The rate of oxygen consumption is -5.9 E-17 mol/[cell◦s]. What is the maximum cellularity (in cells/mL, three significant digits) that this tissue engineered skin can support?arrow_forwardMike has determined that enzyme he is attempting to purify has an isoelectric point of 4.5 (pI = 4.5). He has decided to examine anion exchange chromatography as a potential purification step. He tested out using 2 different buffer and linear NaCl gradient on HPLC (like what you did). His results are shown below. Which buffer should Mike uses for his purification (both buffer has pH higher than enzyme’s pI)? Why?arrow_forward
- Absorbance is directly proportional to glucose concentration for both routine clinical glucose spectrophotometry methods: glucose oxidase method and the hexokinase method. For each one, name the final product measured in each test reaction, which is proportional to the sample glucose concentration. A.) glucose oxidase: B.) hexokinase:arrow_forwardThis is a visible spectra between 390-590 nm obtained during the protein separation process of haemoglobin and cytochrome c using CM Sephadex chromatography. I'd like the results shown on the image interpreted. Look for characteristic peaks or patterns that correspond to the absorption properties of these proteins in the visible range.arrow_forwardThe Bradford reagent gives a linear response only from 0.1 mg/mL to 1.4 mg/mL of protein concentration. Would we be able to use an "absorbance vs. concentration" line graph for samples outside of the range indicated? Justify your response.arrow_forward
- Given this, if you used 6g of vitamin Z powder to make 20 ml of solution, what is the % concentration of this solution? (I gave the image since I don't know if that info is needed to solve this question.)It also gives a follow-up, if you can help here too: You work in a lab as a summer student. One of your tasks is to make sure that there is enough cell culture medium containing antibiotics to grow bacteria. One day you realize that there is only 5 ml of 10% Antibiotic stock solution in the freezer. You decide to use it all to prepare the working culture medium with 0.01% antibiotic. In the lab there is plenty of growth medium without antibiotics. (Note: dilution in medium is like dilution in water). You remember the equation to make dilutions of stock solutions. You usually use this formula to calculate the required volume of a stock solution, but you realize it can apply here as well, even though the unknown is the final volume. So, you make that dilution. Given that each bacterial…arrow_forwardFind a method that uses some form of HPLC for the analysis of proteins. What was the stationary phase used? How does this kind of stationary phase separate the proteins? What kind of mobile phase was used? Was the method isocratic or was a gradient used? How were the proteins detected?arrow_forward1.0 0.9 Z 0.8 15. A pharmaceutical company studied the binding of three different compounds, X, Y, and Z, to a particular protein of interest. For each compound, the fractional saturation (v) was determined as a function of concentration. The results are shown in the figure. Which of the three compounds has the strongest affinity for the protein? Explain. 0.7 X 0.6 > 0.5 0.4 0.3 0.2 0.1 0.0 Y 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 2.2 2.4 2.6 2.8 3.0 L(μM)arrow_forward
- Which fractions measured from your gel filtration experiment had the most and least amount of protein detected? Which fraction likely contained the most myoglobin based off molecular weight and protein presence? Which fraction likely contained the most egg albumin based off molecular weight and protein presence? Which samples measured from your ion exchange experiment had the most protein detected? Calculate the approximate pI of your proteins using the ion exchange Table 2. Ion Exchange Samples Tested by Bradford Assay Concentration BSA (μg/mL) Concentration BSA (mg/mL) Volume of Sample added to Bradford Reagent (μL) Absorbance at 595 nm Standard 1 250 0.25 50 0.32 Standard 2 125 0.125 50 0.16 Standard 3 62.5 0.0625 50 0.08 Standard 4 31.25 0.03125 50 0.04 Standard 5 15.625 0.015625 50 0.02 Blank 0.00 0.00 0.00 Table 3. Gel Filtration Fractions Tested by Bradford Assay Fraction #:…arrow_forwardIn a nutrient medium that lacks histidine, a thin layer of agar containing ~109 Salmonella typhimurium histidine auxotrophs (mutant cells that require histidine to survive) produces ~13 colonies over a two-day incubation period at 37 ° C. How do these colonies arise in the absence of histidine? The experiment is repeated in the presence of 0.4 μg of 2-aminoanthracene. The number of colonies produced over two days exceeds 10,000.What does this indicate about 2-aminoanthracene? What can you surmise about its carcinogenicity?arrow_forwardA sample of a protein is analyzed by CE using a neutral-coated capillary with a total length of 25.0 cm and a distance to the detector of 22.0 cm. Using an applied voltage of 20.0 kV, the protein was detected at a migration time of 16.2 min. The diffusion coefficient for the protein is 2.4 x 10-7 cm?/s. (a) Calculate the apparent electrophoretic mobility of the protein. (b) Calculate the expected number of theoretical plates for the analysis.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON