
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
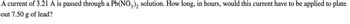
Transcribed Image Text:A current of 3.21 A is passed through a Pb(NO3)2 solution. How long, in hours, would this current have to be applied to plate
out 7.50 g of lead?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: MnO₂ (s) + 4H* (aq) + 2Cr²+ (aq) → Mn²+ (aq) + 2H₂O (1) + 2Cr³+ (aq) 2+ 2+ 3+ Suppose the cell is prepared with 3.73 MH* and 1.42 M Cr²+ in one half-cell and 4.21 M Mn²+ and 3.05 M Cr³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 1 x10 X μ 00 Śarrow_forwardA current of 3.68 A is passed through a Sn(NO3)2 solution. How long, in hours, would this current have to be applied to plate out 5.40 g of tin?arrow_forward4arrow_forward
- A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2VO₂ (aq) + 4H* (aq) + Fe (s) → 2VO²+ (aq) + 2H₂O (1)+Fe²+ (aq) Suppose the cell is prepared with 3.37 M VO and 3.31 MH* in one half-cell and 6.13 M VO²+ and 2.52 M Fe²+ in the other. 2+ 2+ Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.arrow_forwardwhat mass, in g, of copper will be deposited from a solution of Cu^2+ by a current of 2.43 A in 7.52 h?arrow_forwardA galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2+ 2+ Cu²+ (aq) + Zn (s) → Cu (s) + Zn²+ (aq) 2+ Suppose the cell is prepared with 5.43 M Cu²+ in one half-cell and 3.33 M Zn in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 0 x10 X μ 00 Śarrow_forward
- A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2+ 2103 (aq) +12H* (aq) + 5Co (s) → 1₂ (s) + 6H₂O (1) +5Co²+ (aq) 2+ Suppose the cell is prepared with 5.23 M IO3 and 2.31 M H in one half-cell and 6.84 M Co in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.arrow_forwardWhat mass of Molybdenum (Mo) could be deposited by electrolysis of an aqueous solution of Mo2(SO4)3 for 125 min using a constant current of 12.0 A?arrow_forwardA galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 3+ 2+ 2 Cr³+ (aq) + 3Ca (s) → 2Cr (s) + 3 Ca²+ (aq) 3+ 2+ Suppose the cell is prepared with 2.68 M Cr in one half-cell and 7.85 M Ca in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. x10 ロ・ロ × μ 0|0 Śarrow_forward
- Calculate the voltage of the redox reaction of element X (group 2) if the Gibb’s free energy evolved during the redox reaction is + 150 kJ/mol. (Faraday constant, 9.649 x 104C)arrow_forwardA galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2Cr³+ (aq) + 3 Ca(s) → 2Cr(s) + 3Ca²+ (aq) Suppose the cell is prepared with 6.35 M Cr3+ in one half-cell and 0.492 M Ca²+ in the other half. Calculate the cell voltage under these conditions. Ered red= -0.744 V Cr³+ (aq) + 3e → Cr(s) Ca²+ (aq) + 2e → Ca(s) Ered= -2.868 V The standard cell potential (E°) for this galvanic cell is [Select] There are [Select] galvanic cell. Under these conditions, the reaction quotient is [Select] volatage is [Select] moles of electrons transferred during to operate this VV. In order to increase the cell voltage for this cell, [Select] V. ✓and the cell >arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY