Question
Needs Complete typed solution with 100 % accuracy.
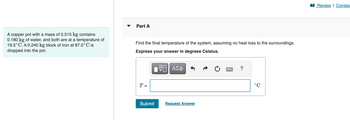
Transcribed Image Text:A copper pot with a mass of 0.515 kg contains
0.180 kg of water, and both are at a temperature of
19.5°C. A 0.240 kg block of iron at 87.0°C is
dropped into the pot.
Part A
Find the final temperature of the system, assuming no heat loss to the surroundings.
Express your answer in degrees Celsius.
T =
——| ΑΣΦ
VE
Submit
Request Answer
Review | Constar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios