
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lijkPWvZoZLqKt1FLlq7wcPWKzBYGfE9IMFjiQ0kz9QM82nFtjswel
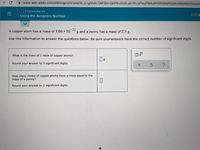
O STOICHIOMETRY
Using the Avogadro Number
-22
A copper atom has a mass of 1.06 × 10
g and a penny has a mass of 2.5
g.
Use this information to answer the questions below. Be sure your answers have the correct number of significant digits.
What is the mass of 1 mole of copper atoms?
g
Round your answer to 3 significant digits.
How many moles of copper atoms have a mass equal to the
mass of a penny?
Round your answer to 2 significant digits.
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please answer question number 2 correctly with the correct answer. there is a picture of an example of a ''Dimensional analysis Format' below. please write out all work showing the math in dimensional analysis format. (please show all work) Question 8: Calculate the mass in grams of each of the following: C.) 0.00100 mol silicon tetrahydride (SiH4) D. ) 1.50 x 10^-2 mol molecular oxygen (O2) E. 2.30 mol ethylene glycol (C2H6O2)arrow_forwardA 81.15 g sample of a compound containing potassium, chromium and oxygen is analyzed as containing 21.57 g potassium and 28.69 g of chromium.. What mass of oxygen is contained in 287.1 g of this compound? Express your answer in decimal notation rounded to the correct number of significant figures.arrow_forwardMass of one a mu)Carbon Type 1 12.0Carbon Type 2 13.0 What is the average mass of carbon in this sample? Is this number on the periodic table?( it may be rounded off ) where?arrow_forward
- Znnznznzzarrow_forwardDr. Dahm has a molecule that has a molecular formula of C18H10O2N3. If he has 12.67 mL of the molecule and its density is 0.842 g/mL calculate how many atoms there are of each type.arrow_forwardAn aluminum atom has a mass of 4.48 x 10 - 23 g and a small airplane has a mass of 5000. kg. Use this information to answer the question below. Be sure your answers have the correct number of significant digits. What is the mass of 1 mole of aluminum atoms? x10 Round your answer to 3 significant digits.arrow_forward
- Determine the number of each type of atom in each of the following formulas. Part A NH4Cl Express your answers as integers. Enter your answers numerically separated by commas. NN, NH, NC1 = = Submit Part B ΠΙ ΑΣΦ Request Answer NMg, Np, No = Submit Mg3(PO4)2 Express your answers as integers. Enter your answers numerically separated by commas. ΠΫΠΙ ΑΣΦ ? Request Answer ?arrow_forwardHow many gold atoms are in an 0.214-ounce, 18 K gold bracelet? (18 K gold is 75% gold by mass.) Express your answer using two significant figures.arrow_forwardAn iron atom has a mass of 9.27 × 10 - 23 g and a cooking pot has a mass of 0.500 kg. Use this information to answer the questions below. Be sure your answers have the correct number of significant digits x10 What is the mass of 1 mole of iron atoms? 5.58 x 10 g How many moles of iron atoms have a mass equal to the mass of a cooking pot? .900arrow_forward
- How many grams of phosphorus are in 2.59 kg of P2O5? Express your answer in units of grams using at least three significant figures. Please type answer not write by hendarrow_forwardHow many moles are there in 1.0 g of the element Al? Your answer must contain the correct number of significant figures. 13 6.022 x 10^23 0.037 4 x 10^-2 1.0arrow_forwardDetermine the mass of the product from the following data. Mass Aluminum Foil (g) 6.3284 Mass Filter Paper (g) 0.2489 Mass Filter Paper + Product (g) 86.2751 Mass Product (g) Group of answer choices 86.0263 g 86.0262 g 86.0260 g 86.0264 g 86.0261 garrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY