
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
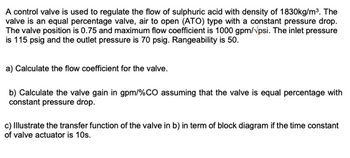
Transcribed Image Text:A control valve is used to regulate the flow of sulphuric acid with density of 1830kg/m³. The
valve is an equal percentage valve, air to open (ATO) type with a constant pressure drop.
The valve position is 0.75 and maximum flow coefficient is 1000 gpm/psi. The inlet pressure
is 115 psig and the outlet pressure is 70 psig. Rangeability is 50.
a) Calculate the flow coefficient for the valve.
b) Calculate the valve gain in gpm/%CO assuming that the valve is equal percentage with
constant pressure drop.
c) Illustrate the transfer function of the valve in b) in term of block diagram if the time constant
of valve actuator is 10s.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 6 images

Knowledge Booster
Similar questions
- 3.53. WP Two mercury manometers, one open-end and the other sealed-end, are attached to an air duct. The reading on the open-end manometer is 25 mm and that on the sealed-end manometer is 800 mm. Determine the absolute pressure in the duct, the gauge pressure in the duct, and the atmospheric pressure, all in mm Hg.arrow_forward*1.12 Water is flowing through a 2-inch diameter pipe with a velocity of 3 ft/s. (ft) (Ib;), (a) What is the kinetic energy of the water in (lbm) (b) What is the flowrate in gal/min?arrow_forwardAnswers- a) V1= 19.9 ft/ s; V2 = 79.7 ft/ s; m = 103.4 lbm/s b)F=288 lbfarrow_forward
- Back HW#2 QUESTION1/60pts] A fluid of viscosity u flows in the horizontal cylinder (radius R) shown in the figure under a constant pressure gradient dP/dx. The inner core of the cylinder is filled with a porous material. The flow in this porous region is slow and assumed to be a plug-type flow such that the velocity is constant and everywhere the same inside the porous region. Denote this velocity by Uo. The flow in the open (non-porous) region is steady, Newtonian, incompressible and axisymmetric. It will be assumed that only the axial (x) component of the velocity is non-zero. Flow Open flow T&R Porous media flow R N.B. All your answers must be expressed in terms of µ, U₁, a, R and dP/dx. (a) Use the continuity and Navier-Stokes equations to determine the expression of the velocity in the open region. (b) What is the expression of the average velocity in the open region? (c) Is the assumption of a linear velocity profile in the open region acceptable when this region Tuatifi 1:arrow_forwardP.15.4 Glycerine (sp. gr = 1.11) is pumped through a heat exchanger and a control valve. At normal conditions, the flow rate is 200 gallons per min., the pressure drop across the control valve is 5 psi while the total pressure drop is 35 psi, and f(x) for the valve is 0.5. Determine the flow rate and the pressure drop across the control valve at the maximum condition.arrow_forwarderical pa for steady FLUID Flow Q: Yotameter with float shape type "B" (see figure below) has tube of 0.3 m long. the internal diameter of the Yota meter is 25 mm at the P and 20mm at the bottom the climmeter of the float is 20mm its elensity is Bl00g/2 and its volume is 5.0 cm² what is the mass flow rate of water (at 25 ℃) When the float Position 0-2 m from the bottom of the tube ? isarrow_forward
- Fluid Mechanics Quiz 5 Q1: Air at 200 °F and 60 psia flows in a 4-in.-diameter pipe at a rate of 0.52 lb/s. Determine the pressure at the 2-in.-diameter throat of a Venturi meter placed in the pipe. Q2: Water flows through a 40-mm-diameter nozzle meter in a 75-mm-diameter pipe at a rate of 0.015 m/s. Determine the pressure difference across the nozzle if the temperature is (3) 10 °C. or (b) 80 °c. Q3: Water flows through the orifice meter shown in Fig. below at a rate of 0. 10 cfs. If d = 0.1 ft, determine the value of h. Q- 2 in.arrow_forwardThe 9000 kg/hr of water is flowing through the steel pipe network. The density and viscosity of water is 998 kg/m3 & 0.001 kg/m.s. The inside diameter of the pipe is 50 mm and the total length of the pipe is 80 m. The velocity of the pipe is 25 m/s. During the pipe network, the total number of valves are 8 Globe valve & 2 90-degree Elbows (standard) and 1-foot valve. Calculate the total pressure drop in the pipe network line?arrow_forwardA ventilation opening in a room is 2 feet by 3 feet. Air flow out of the opening is measured at 75 feet per minute. Calculate the CFM rate.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The