Question
Transcribed Image Text:20 of 20
I
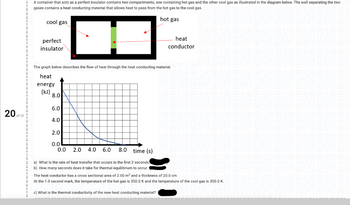
A container that acts as a perfect insulator contains two compartments, one containing hot gas and the other cool gas as illustrated in the diagram below. The wall separating the two
1
gases contains a heat conducting material that allows heat to pass from the hot gas to the cool gas.XO OXO OXO OXO OXO OXO O
1
1
hot gas
FENN FENNEEN
cool gas
perfect
insulator
erial.
The graph below describes the flow of heat through the heat conducting material.
heat
energy
(kJ) 8.0
NC
6.0
4.0
1
2.0
1
0.0
I
0.0 2.0 4.0 6.0 8.0 time (s)
14
1
a) What is the rate of heat transfer that occurs in the first 2 seconds.
b) How many seconds does it take for thermal equilibrium to occur.
The heat conductor has a cross sectional area of 2.00 m² and a thickness of 20.0 cm.
103000:
1
At the 1.0 second mark, the temperature of the hot gas is 350.0 K and the temperature of the cool gas is 300.0 K.
c) What is the thermal conductivity of the new heat conducting material?
heat
conductor
OKROVO
ENV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Which of the following is NOT correct regarding open or closed systems? Select one: a. Both mass and energy can cross the boundary of a control in an open system O b. no mass can cross the boundary in a closed system O c. A closed system is not a fixed mass system d. heat and work can cross the boundary in a closed systemarrow_forwardA 1kg of metal requires 1080 Joules of energy to have a 1 degree Celsius change in temperature. What is the constant value described in the statement? * the latent heat is 1080 J/kg the coefficient of linear expansion is 1080 /C² O the specific heat capacity is 1080 J/kg.Cºarrow_forward20. The kinetic molecular model of an ideal gas shows that * 1 average translational kinetic energy of a molecule is inversely proportional to the absolute temperature average translational kinetic energy of a molecule is directly proportional to the absolute temperature average translational kinetic energy of a molecule is not dependent to the absolute temperature average translational kinetic energy of a molecule is equal to the absolute temperature 21. The first law of thermodynamics helps us understand the relationships 11 among which three quantities? Heat, work, and internal energy O Heat, work, and external energy O Heat, work, and entropy O Heat, work, and enthalpyarrow_forward
- The latent heat of vaporisation of water is 2,240 J. If the work done in the process of vaporisation of 1g is 168 J, then the increase in internal energy is? a) 2408 J b) 2240 J c) 2072 J d) 1904 Jarrow_forwardQuestion 2arrow_forward20°C 25°C During day time, water is colder than the sand. Which has higher specific heat capacity, water or sand? * O Incomplete information is given. O Sand, as increases its temperature increases easily. Sand, as it needs less energy to increase its temperature O Water, as it needs more energy to increase its temperature.arrow_forward
- Question 5 During an sothemal process, 5.0J of heat is removed from an ideal gas. What is the change in internal (thermal) energy of the gas? O 25 J O 25 J O BL9J O 30Jarrow_forward* Your mass is 56 kg and eat 700-calorie food, calculate how high of stairs you must climb to work off the energy from your food eaten. A. 5334 m B. 6410 m C. 10189 m D. None above A. В. C. D.arrow_forwardPlease don't provide handwritten solution...arrow_forward
- 2.arrow_forward9,3 skill application The heating curve below shows the change in temperature of a 20 g sample of a substance as energy is added to the substance as heat. (Note that the horizontal scale of the graph is not uniform.) 600 Gas E 450 Liquid + gas Solid + Liquid liquid 300 Solid 150 1.85 12.0 16.6 855 857 Heat (kJ) d. the specific heat capacity of the vapor e. the latent heat of vaporization Temperature (°C)arrow_forwardIn reference to the heat curve found below, which of the following statements is false? Temperature (C) Energy Goined (7) The object is gaining energy for the entire portion of the curve The object undergoes one state change in the process O The object's final temperature was higher than it's initial temperature O The object started in a solid state The object finished in a gas statearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios