
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:(a) Consider the reactions to be liquid phase and plot the species concentrations and the conversi
of A as a function of the distance (i.e., volume) down a 50-dm³ PFR. Note any maxima
(b) Consider the reactions to be liquid phase and determine the effluent concentrations and conversion fro
a 50-dm CSTR. (Ans.: C = 0.61, C, = 0.79, CF = 0.25, and Cp = 0.45 mol/dm3.)
%3D
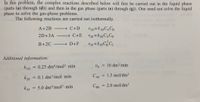
Transcribed Image Text:In this problem, the complex reactions described below will first be carried out in the liquid phase
(parts (a) through (d)) and then in the gas phase (parts (e) through (g)). One need not solve the liquid
phase to solve the gas-phase problems.
The following reactions are carried out isothermally.
A+2B - C+D
2D+3A
→ C+E
CH
B+2C
→ D+F
Additional information:
kID
0.25 dm6/mol2 - min
vo = 10 dm/min
%3D
k2E
= 0.1 dm3/mol min
CAO
= 1.5 mol/dm³
5.0 dm/mol2 min
CBo = 2.0 mol/dm³
k3F
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- 1008 r_max is right need k_max but also how?arrow_forwardReaction rate is expressed in terms of changes in concentration of reactants and products. Drag and drop the appropriate choices to fill in the correct coefficients to balance the equation for the reaction with this rate expression: Rate = -4/3 A[B]/At = A[C]/At = -1/2 A[A]/At = 2 A[D]/At Equation to balance: A + B Darrow_forwardWhat will be the molecular diffusion coefficient of naphthalene vapor in air at 0 °C and 1 Atm pressure;a) Chapman-Enskogb) Estimate from Fuller, Schettler and Giddings (Atomic diffusion volumes) Equations. Compare the values you found with the experimental value of 0.0513 cm2 /s.The critical temperature and volume of naphthalene are 748.4 K and 408 cm3/gmol. The critical temperature and volume of air are 132.5 K and 90.52 cm3 /gmol.MNaphthalene=128.16 and MHair=29arrow_forward
- Thermodynamics of Warfarin binding to human plasma albumin.Warfarin (coumadin) binds to human plasma albumin to prevent blood clotting in the reactionMeasured thermodynamic values for this reaction at 25oC are ∆G =-30.8 kJ/mol, ∆H=-13.1 kJ/mol, and ∆Cp~0.(a) Determine the entropy change for this reaction at 25oC.(b) Determine the fraction of unbound albumin over a temperature range of 0 to 50oC for a solution initially containing warfarin and albumin at 0.1 mM.arrow_forwardfeedback to help with ques: Q4. Again, we know that the molar flux to and from the interface must be equal (no accumulation), and that there is equilibrium at the interface. Knowing the bulk mole fractions and film mass transfer coefficients allows us to solve for the other unknowns, including the compositions of vapour and liquid at the interface, y* and x*. A graphical approach is required since we are given the equilibrium line as a plot. ans Q4 XA *=0.28; yA *=0.67; NA=0.006 mol m-2 s-1 ans 4) A packed distillation column is used to separate methanol from water. At an intermediate height within the packed column, the mole fraction of methanol in the bulk of the vapour is 0.6 and the mole fraction of methanol in the bulk of the liquid is 0.4. Using the xy diagram overleaf, determine the molar flux of methanol from liquid to vapour at this vertical position in the column, given the film mass transfer coefficients, KG=0.1 mol m² s-1 and kL=0.05 mol m-² s-¹.arrow_forwardQ.1) In an air-CO2 mixture at 298k & 303.9 KN/m², the concentration of CO2 at two planes (2mm) apart are 20% & 30%. The diffusivity of CO2 under the 273 k and 101.3 KN/m² is (1.85 x 104 m²/s), calculate the rate of transfer of CO2 across the two planes assuming; a. Diffusion of CO2 through stagnant air layer . b. Equimolar counter diffusionarrow_forward
- Given the following cell Pb, PbCl2; HCl (1m); AgCl,AgThe value of the electromotive force at 25oC is 0.4900 volt. The rate of change of the electromotive force with temperature is 0.000186 volt/degree. Calculate ∆H, ∆S, and ∆G for the reaction.arrow_forwardThe following questions are in regard to the mass transfer a.) If you have a system with the same soulute concentration everywhere, will mass transfer occur? b.) When will a bulk concentration equal an inerfacial concentration? When won't it? c.) What are typical units for partition coefficients?arrow_forward8arrow_forward
- Experiments are conducted on the uptake of a water pollutant in water over an adsorbing solid. Once equilibrium is reached the concentration C, of the pollutant in the water (in mg/L) and the ratio Q of mass of adsorbing solid/mass of adsorbed pollutant (in g/mg) are measured. Experimental data are presented in the table below. These data are correlated using the following equation: where a and 8 are constants and: Cp is in mg/L Q is in g/mg a. Determine the units of oa and B in this equation: b. Appropriately transform the above equation using newly defined variables so that you can linearly regress the data visually to obtain the values of a and B. Cp (mg/L) Q (g/mg) 0.0049 1.3 0.0035 12.5 0.0027 75.2 0.0018 930.2arrow_forwardPlease help me only 7,8,9,10 please....arrow_forwardGive two reasons (short statements) why the rate of a catalyzed reaction mightdecrease as the pressure of one of its products increases.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The