
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
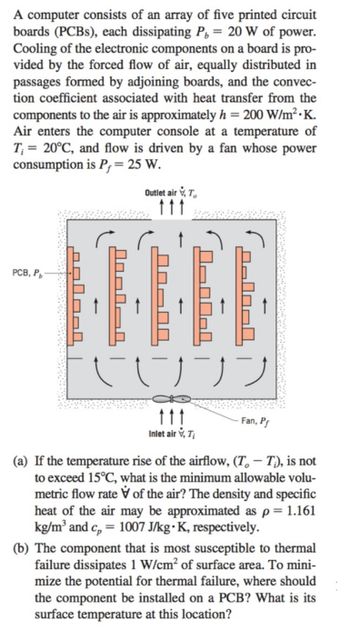
Transcribed Image Text:A computer consists of an array of five printed circuit
boards (PCBs), each dissipating P, = 20 W of power.
Cooling of the electronic components on a board is pro-
vided by the forced flow of air, equally distributed in
passages formed by adjoining boards, and the convec-
tion coefficient associated with heat transfer from the
components to the air is approximately h = 200 W/m².K.
Air enters the computer console at a temperature of
T₁= 20°C, and flow is driven by a fan whose power
consumption is P = 25 W.
PCB, Pb
salool
Outlet air, T
J-00-1
090040
UJ JJ
ttt
Inlet air, Ti
Fan, Pf
(a) If the temperature rise of the airflow, (T — Tì), is not
to exceed 15°C, what is the minimum allowable volu-
metric flow rate of the air? The density and specific
heat of the air may be approximated as p = 1.161
kg/m³ and c = 1007 J/kg • K, respectively.
(b) The component that is most susceptible to thermal
failure dissipates 1 W/cm² of surface area. To mini-
mize the potential for thermal failure, where should
the component be installed on a PCB? What is its
surface temperature at this location?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 7 images

Knowledge Booster
Similar questions
- A shaft of 0.06-m diameter is heat treated in a gas-fired furnace whose gases is at 1200 K and provide a convection coefficient of 125 W/m² K. If the shaft enters the furnace at 300 K and stays in the furnace. (1) What is the centerline temperature (in K) of the shaft after 900 seconds? (2) What is the value of the dimensionless Fourier number? The shaft material has a density of 7000 kg/m², thermal conductivity of 50 W/m.K, and specific heat capacity 500 J/kg.K. Please show your work and assumptions.arrow_forward3 An electrical current of 700 A flows through a stainless steel cable having a diameter of 5 mm and an electrical resistance of 6 x 104 /m (i.e., per meter of cable length). The cable is in an environment having a tem- perature of 30°C, and the total coefficient associated with convection and radiation between the cable and the environment is approximately 25 W/m².K. (a) If the cable is bare, what is its surface temperature? (b) If a very thin coating of electrical insulation is applied to the cable, with a contact resistance of 0.02 m² K/W, what are the insulation and cable surface temperatures? (c) There is some concern about the ability of the insula- tion to withstand elevated temperatures. What thick- ness of this insulation (k = 0.5 W/m .K) will yield the lowest value of the maximum insulation temper- ature? What is the value of the maximum tempera- ture when this thickness is used? Note: The 25 W/m²K includes not only convection but also radiation.arrow_forwardA thin metal plate 1 mx 1 m is placed on a rooftop. It receives radiant heat from the sun directly at the rate of 170 W/m². If heat transfer from the plate to the ambient occurs purely by free convection, calculate the steady state temperature of the plate. Assume that there is no heat loss from the bottom of the plate. The ambient temperature is 25°C.arrow_forward
- À wall of area 30 m² having a density of 1500 kg/m', thermal conductivity 30 W/m.K, and specific heat capacity 4 kJ/kg.K. The temperature distribution across a wall 0.5 m thick at a certain instant of time is given as T(x) = 30-5 x-7x The wall is generating a uniform heat (q.) of 1000 W. (1) Find the rate of heat transfer entering and leaving the wall (in W). (2) Find rate of energy stored in Watt. (3) Find (dFT/dx²) (4) Derive the change in temperature with respect to time equation (time rate of temperature change)- remember to substitute the value of (d T/dx²) from (part 3) and values of all other properties into final equation. %3Darrow_forwardSteam enters a heat exchanger operating at steady state at 2 bar with a specific enthalpy of 1845 kJ/kg and exits at the same pressure as a saturated liquid. The steam mass flow rate is 1.6 kg/min. A separate stream of air with a mass flow rate of 68.5 kg/min enters at 37 °C and exits at 66.3 °C. The ideal gas model with c, = 1.005 kJ/kg.K can be assumed for air. Kinetic and potential energy effects are negligible.arrow_forwardTransport Phenomena Questionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The