
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN: 9781305079250
Author: Mark S. Cracolice, Ed Peters
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
How can we find out the formula?
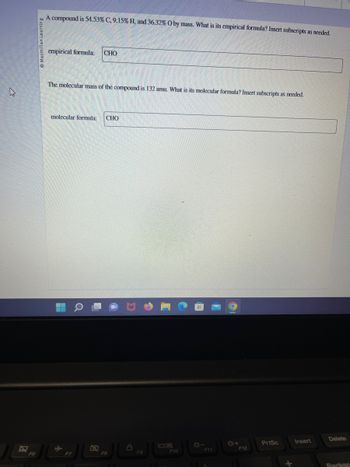
Transcribed Image Text:**Problem: Determining Empirical and Molecular Formulas**
A compound is composed of 54.53% Carbon (C), 9.15% Hydrogen (H), and 36.32% Oxygen (O) by mass. Determine the empirical formula of the compound. Insert subscripts as needed.
**Empirical Formula:**
CHO
---
The molecular mass of the compound is 132 amu. Determine the molecular formula. Insert subscripts as needed.
**Molecular Formula:**
CHO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A compound is composed of 60.00% carbon, 32.00% oxygen, and 8.00% hydrogen. Determine its empirical formula, writing the atoms in the molecule in the same order they appear in the problem.arrow_forwardIt may be said that because atomic, molecular, and formula masses are all based on carbon-12, they are conceptually alike. What then are their differences?arrow_forward2.37 Why are empirical formulas preferred for describing polymer molecules?arrow_forward
- Use percent composition and molar mass to determine molecular formula. Close Problem Question Content Area Analysis of a compound of chlorine, oxygen and fluorine showed that it is 34.60% Cl and 46.85% O, with F accounting for the remainder. In a separate experiment, the molar mass of the compound was found to be 102.5 g/mol. Determine the molecular formula of the compound. Enter the elementsin the order Cl, O, F.arrow_forwardQuestion attachedarrow_forwardProblem A sample of an unknown compound contains 0.21 mol of zinc, 0.14 mol of phosphorus, and 0.56 mol of oxygen. What is the empirical formula?Plan We are given the amount (mol) of each element as fractions. We use these fractional amounts directly in a preliminary formula as subscripts of the element symbols. Then, we convert the fractions to whole numbers.arrow_forward
- Use percent composition and molar mass to determine molecular formula. Close Problem Question Content Area Analysis of a compound of carbon, hydrogen and oxygen showed that it is 59.94% C and 13.44% H, with O accounting for the remainder. In a separate experiment, the molar mass of the compound was found to be 60.11 g/mol. Determine the molecular formula of the compound. Enter the elementsin the order C, H, O.arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardchoose an ionic compound that contains a polyatomic ion. state the name and the correct chemical formula using subscripts as appropriate, then show work for calculating its molar massarrow_forward
- The name of the compound shown here is 'Narrow_forwardProblem Ammonium carbonate is a white solid that decomposes with warming. It has many uses, for example, as a component in baking powder, fire extinguishers, and smelling salts.(a) How many formula units are in 41.6 g of ammonium carbonate?(b) How many O atoms are in this sample?Plan (a) We know the mass of compound (41.6 g) and need to find the number of formula units. we need the formula to find the amount (mol) and then multiply by Avogadro’s number to find the number of formula units. [The road map steps are for part (a).] (b) To find the number of O atoms, we multiply the number of formula units by the number of O atoms in one formula unit.arrow_forwardHelp!!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
