
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Provide the total electron count, valency, and dn configuration for each metal center
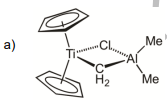
Transcribed Image Text:a)
-CL
Ti:
of
C
Al
Me
Me
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 0.0712-g sample of a purified organic acid was dissolved in an alcohol-water mixture and titrated with coulometrically generated hydroxide ions. With a current of 0.0392 A, 241 s was required to reach aphenolphthalein end point. Calculate the equivalent mass of the acid.arrow_forwardWrite the chemical equationarrow_forward5. A 17.1769 mL sample of an acid was titrated with electrogenerated OH-. It took 617s at a constant current of 0.216A to reach a phenolphthalein end point. Determine the molarity of the acid.arrow_forward
- What happens when too much NaSCN is prepared in solution of mixtures of standard solutions of Fe(NO3)3 and NaSCN, so that [SCN^-] is higher than expected? What does this do to the measured Keq?arrow_forwardWhat are the respective concentrations (M) of Fe3+ and I- afforded by dissolving 0.200 mol FeI3 in water and diluting to 725 mL? A) 0.276 and 0.828 B) 0.828 and 0.276 C) 0.276 and 0.276 D) 0.145 and 0.435arrow_forward-6 -5 -4 -3 -2 -1 +1 +2 +3 +4 +5 PbsO.(s) + H*(aq) + 2CI (aq) = Pb(s) + HSO. (aq) + Cl2(g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning