
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
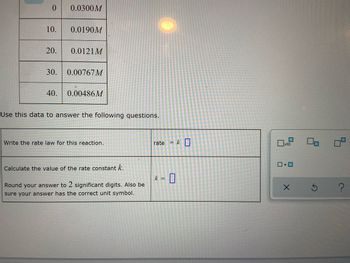
A chemistry graduate student is studying the rate of this reaction:
She fills a reaction vessel with and measures its concentration as the reaction proceeds:
time
(seconds)
Use this data to answer the following questions.
Write the rate law for this reaction. rate
Calculate the value of the rate constant .
Round your answer to significant digits. Also be sure your answer has the correct unit symbol.
![E
Deducing a rate law from the change in concentrati
er time
A chemistry graduate student is studying the rate of this reaction:
2 HI(g) → H₂(g) + 1₂ (g)
She fills a reaction vessel with HI and measures its concentration as the reaction proceeds:
time
(seconds)
[HI]
0
0.0300M
10. 0.0190M
20. 0.0121M
30.
0.00767M
40.
0.00486M
Use this data to answer the following questions.
x10
Write the rate law for this reaction.
0.0
Calculate the value of the rate constant k.
Explanation
Check
rate = k
Lis Danonind
Terms of](https://content.bartleby.com/qna-images/question/7753cf2d-6495-4e63-80ab-6916bfb5226b/1459d6a1-88a3-4d37-ba50-092381593df9/jmb5e8_thumbnail.jpeg)
Transcribed Image Text:E
Deducing a rate law from the change in concentrati
er time
A chemistry graduate student is studying the rate of this reaction:
2 HI(g) → H₂(g) + 1₂ (g)
She fills a reaction vessel with HI and measures its concentration as the reaction proceeds:
time
(seconds)
[HI]
0
0.0300M
10. 0.0190M
20. 0.0121M
30.
0.00767M
40.
0.00486M
Use this data to answer the following questions.
x10
Write the rate law for this reaction.
0.0
Calculate the value of the rate constant k.
Explanation
Check
rate = k
Lis Danonind
Terms of
Transcribed Image Text:0
0.0300M
10.
0.0190M
20.
0.0121M
30. 0.00767 M
40. 0.00486M
Use this data to answer the following questions.
Write the rate law for this reaction.
Calculate the value of the rate constant k.
Round your answer to 2 significant digits. Also be
sure your answer has the correct unit symbol.
rate = k
k = 0
0
x10
0.0
X
S
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- * Question Completion Status: A Moving to another question will save this response. Question 18 The mechanism for NO2 (g) + CO (g) → NO (g) + CO2 (g) is thought to be: 2 NO2 → NO3 + NO NO3 + CO → NO2+ CO2 (slow) (fast) The rate law for the overall reaction is: Rate = k[NO3][CO] O Rate = k[NO2] Rate = k[NO2]2 O Rate = k[NO2][CO] O Rate = k[NO3][NO] A Moving to another question will save this response. MacBook Air 吕0 F6 esc F3 F4 F5 F1 F2 #3 $4 % 3 4. 6. 回2 Breathe easyarrow_forwardA chemistry graduate student is studying the rate of this reaction: CICH, CH,CI (g) → CH,CHCI (g) + HC1 (g) She fills a reaction vessel with CICH,CH,Cl and measures its concentration as the reaction proceeds: [CICH,CH,CI] time (minutes) 0.600M 1.0 0.411 M 2.0 0.281 M 3.0 0.192M 4.0 0.132M Use this data to answer the following questions. Write the rate law for this reaction. rate = k Ox10 Calculate the value of the rate constant k. k = Round your answer to 2 significant digits. Also be のarrow_forwardAn elementary step is defined as a chemical collision in a reaction mechanism. A collection of different types of collisions makes up the reaction mechanism, so elementary steps provide a molecular view of the overall reaction. Write the rate law for the following reaction, which represents an elementary step in a reaction. Your rate law should not include the states of matter. $$SO2Cl2(g)SO2(g)+Cl2(g)arrow_forward
- Please answer all parts.arrow_forwardA chemistry graduate student is studying the rate of this reaction: She fills a reaction vessel with and measures its concentration as the reaction proceeds: time (seconds) Use this data to answer the following questions. Write the rate law for this reaction. rate Calculate the value of the rate constant . Round your answer to significant digits. Also be sure your answer has the correct unit symbol.arrow_forwardThe rate of a certain reaction is given by the following rate law: rate =k[N=][H2] Use this information to answer the question below. The rate of the reaction is measured to be 0.210 M / s when [N2] = 0.68 M and [H2] = x10 -1 k = OM 2.0 M. Calculate the value of the rate constant. Be sure your answer has the correct number of significant digits.arrow_forward
- Some measurements of the initial rate of a certain reaction are given in the table below. [N] 0.815M1.56M N,|H||initial rate of reaction 185. M/s 141.M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. 口 rate = 0 ロ || = 1 Explanation Check D2022 McGrny Hl LLC. Al Rights Reserved. Terms of N 回 至 五arrow_forwardThe synthesis of ammonia obeys the following reaction: N2(g) + 3H2(g) → 2 NH3(g) What is the average reaction rate of H2(g) between the 2nd and the 9th hour. (Attention, the graph is in "hour" and we want a speed in "mole/L·s".) What is the instantaneous reaction rate of H2(g) at the 3rd hour. What is the reaction rate between the 2nd and the 9th hour.arrow_forwardDeducing a rate law from initial reaction rate data Some measurements of the initial rate of a certain reaction are given in the table below. [N] [H] initial rate of reaction 0.335 M 2.47M 18.0M/s 0.335 M0.592M 1.03 M/s olo 0.521 M 2.47M 28.0 M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k|| Ix10 k = ||arrow_forward
- A chemistry graduate student is studying the rate of this reaction: NH4OH(aq)—>NH3(aq)+H2O(aq) He fills a reaction vessel with NH4OH and measures its concentration as the reaction proceeds (shown in picture). A. Write the rate law for this reaction B. Calculate the value of the rate constant k. Round your answer to two significant digits. Also be sure your answer has the correct unit symbol.arrow_forward= Using a rate law The rate of a certain reaction is given by the following rate law: rate = k[H₂ ] [NH₂] Use this information to answer the questions below. What is the reaction order in H₂? What is the reaction order in NH3? What is overall reaction order? At a certain concentration of H₂ and NH3, the initial rate of reaction is 17.0 M/s. What would the initial rate of the reaction be if the concentration of H₂ were doubled? Be sure your answer has the correct number of significant digits. The rate of the reaction is measured to be 2.0 x 10³ M/s when [H₂] = 0.75 M and [NH₂] = 0.44 M. Calculate the value of the rate constant. Be sure your answer has the correct number of significant digits. k= 0 0 04/ -1 X 1/5arrow_forwardDeducing a rate law from initial reaction rate data Some measurements of the initial rate of a certain reaction are given in the table below. [N] [H] initial rate of reaction 0.977 M 1.26 M 0.162 M/s 2.87 M 1.26 M 1.40 M/s 0.977 M 2.13 M 0.274 M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = kO k = 0 Explanation Check O 2021 McGraw-Hill Education, All Rights Type here to search 立arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY